|
Home > Household Chemical Encyclopedia
Hazardous Household Chemical Products Encyclopedia - Hazards Lurking in Your Home101 Household Chemical Hazards:Welcome to one of the World Wide Web's most extensive guides to common household hazardous materials. I created this resource to help you answer all of the following questions... What exactly is contained within these harmless looking cans, bottles, boxes, and bags? Are these exotic and carefully marketed solids, liquids, and gases really hazards in the home? What might the far-reaching consequences be of a home filled with these many creations of "better living through modern chemistry"? Are these dangerous and toxic ingredients contaminating my home and affecting my family's health or contributing to my allergy symptoms or frequent illness? How can I test for the chemical footprint of my home to know for sure if there is an indoor air quality problem and what the sources might be? How can I safely dispose of and replace many of these dangerous products with safer, non-toxic, green alternatives? 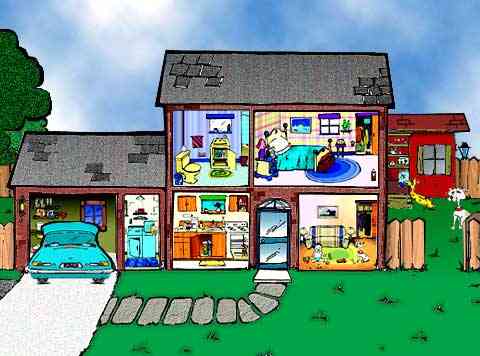
See the link chart of common household chemical products below or click the rooms above to explore the house of chemicals. If you are looking for something specific you may also wish to try the site search box at page bottom. Now let's find out if your home sweet home may be a toxic waste dump...
Don't miss my other website about frugal living where I have an ever-growing extensive list of natural, non-toxic, home-made green cleaning and household products recipes that will help to save your health, save money, and save the planet.
If you suspect something in your home air is making you sick, there is a good chance your symptoms may be caused by what are known as Volatile Organic Compounds (VOCs). Many common household chemical products can greatly increase the levels of VOC inside your home. There can also be hundreds of other VOC sources in the actual structure and decor items in the average home. These VOC sources often continuously off-gass toxic chemical poisons into your indoor environment where they can often accumulate and damage the health of people and pets.
Some examples of VOC sources are: engineered wood products such as particle board or oriented strand board (OSB) which are known to often contain adhesives which give off toxic Formaldehyde. Paints, lacquers, and other solvent-based products such as perfumes and nail polish are also common indoor VOC sources. In our air-tight energy-efficient homes, the toxins tend to accumulate with each use and this can increase our long-term exposure and health damage. Also, you would not believe how many upset tenants living above actively used garages have contacted me because they were being poisoned through the floor boards from below by vehicle exhaust, household hazardous product fumes, or gasoline cans off-gasing noxious vapors - such as leukemia causing Benzene. Gasoline fumes and garage air pollution is a bigger problem than most people realize. It only takes a little gasoline vapor infiltrating regularly to contaminate everything in a room and make a space totally unlivable for quite some time. Of all the indoor air conundrums I have been contacted about, chemicals in one form or another is often the cause of the complaints or mysterious illnesses. And mold spores seems to be the second most common cause. But even with an on-site inspection in those cases, it would often still have been difficult to draw clear conclusions about exact symptom causes unless the air was actually tested to see what contaminants may be elevated. So I always recommend an accurate air test be performed to take the guess work out the equation. Otherwise, indoor allergy symptom causes will often remain a difficult to solve mystery.
Please do not get fleeced by the golden fleecers! I have arranged to have Mr. In-home expert tester cut totally out of the picture. I have made it so homeowners can do their own pro-grade testing and save over 80% and get the same expert results and follow-up recommendations all-inclusive to the low cost of the following very simple to use air test kit. This is the best indoor air quality testing kit many experts use to measure both mold and chemical VOC fumes...but now visitors to this website can get it for a fraction of what the experts would charge you. And if you can flip a switch, you can do it yourself with this kit. No expert required. As you can see on that page, I have arranged an additional special discount code so anyone can get an extra 10% off plus free shipping to and from the certified lab. Everything is included in the cost of it, as compared to how some OTHER flimsy test kits for sale elsewhere will often charge an additional steep fee just for the actual analysis. Don't fall for that please. Those who test with this easy to use top-of-the-line kit usually will have a much better idea what the sources of indoor air pollution may or may not be because they can test for hidden mold colonies, over 400 chemicals, Formaldehyde gas, or cigarette smoke residues. That covers the majors and helps identify exactly what needs to be removed to create a more healthy home.
For parents of newborn babies, the very old, or the already chronically ill I urge you in particular to have your indoor air tested with the above kit.
Now on to the chart of household hazardous chemicals and products.
How Many of These Household Hazardous Waste Sources Are You Stockpiling Inside Your Home? Back to Top
ACETONEAcetone is used in paint, varnish, solvents, and cleaning fluids and is the primary chemical agent used in many nail polish removers. It is an irritant with a characteristic sweet odor. If inhaled, cough and bronchial irritation, along with depressed respirations, may occur. Skin contact can cause peeling and splitting of nails and skin rashes. Visit Our Complete Guide to Acetone for extensive health and MSDS information about the specific Brands containing Acetone, the sources, the occupations, and diseases associated with Acetone. ACIDSAcids are found in several household cleaning compounds, pool chemicals, solvents, wet cell batteries, and radiator flushers and cleaners. Acids, which have a pH range of 0 to 6.9, may be corrosive and produce severe burns on contact. Vinegar, which contains four to six percent acetic acid, is generally considered non-toxic.Skin contact with acid may produce severe pain and risk of secondary infection and scarring. Chronic skin exposure to acids may cause mild irritation, dermatitis, or roughened skin. Inhalation of fumes may produce nose and throat irritation, coughing, chest pain, and even pulmonary edema. The onset of symptoms following inhalation of vapors may be delayed for several hours. When working with household products containing acids, wear protective gloves. Make sure the ventilation is adequate. Refer to the specific product for disposal recommendations.
AEROSOLSAerosol sprays (e.g., furniture polish, deodorant, and air freshener) contain an active ingredient and a liquid or gaseous propellant that is packed under at least 40 pounds of pressure per square inch. These pressurized aerosol containers are explosive and may be flammable. The actual product propelled by the aerosol, such as some oven cleaners, can be corrosive or poisonous, therefore requiring great care. Aerosol sprays should be used with care. The fine particles emitted from aerosol sprays are easily breathed deeply into the lungs and quickly absorbed into the bloodstream. Thus, a chemical that is harmless to your skin may become extremely dangerous if inhaled as a mist. Acute symptoms include headache, nausea, dizziness, shortness of breath, eye and throat irritation, skin rash, burns, lung inflammation, and liver damage. If spray is misdirected, chemical burns and eye injury can also occur. Intentionally inhaling aerosol gases for kicks, sometimes called "sniffing" or "huffing," has resulted in the death of several young Americans. An aerosol container should never be heated significantly above room temperature because it can explode. Storage of cans in direct sunlight, car trunks, and near furnaces, stoves, and ovens can result in explosion. When heated, aerosol gases can turn into toxic gases including fluorine, chlorine, chloride or hydrogen fluoride, or phosgene (military nerve gas). Breathing these vapors can be very harmful to you. Significant environmental impact from aerosol sprays led to alterations in their design. Several of the Chlorofluorocarbons (CFCs) that have been used in aerosol sprays in the past reacted with and reduced the ozone layer in the upper atmosphere. Reduction in the ozone layer and the resulting rise in ultraviolet radiation reaching the earth can result in increased rates of skin cancer, skin aging, eye damage, and Vitamin D poisoning. Before buying or using aerosol sprays, weigh their convenience against their potential health and environmental hazards. Use: Consider alternatives to aerosol sprays, including alternative methods of application. If you are using an aerosol spray, try not to breathe the released particles; stand out of the way of the mist and make certain the mist is being blown away from you. (An exception to this advice is for bronchial or asthma medication dispensed by aerosol spray.) Storage: Do not store near heat or flames. Keep away from children. Disposal: If the aerosol can is empty, dispose of it in the trash bound for the landfill. Aerosol cans burned in trash barrels can explode, scattering propellant and product. If ingredients are left in the can the best thing to do is to use the product up as intended. If you must dispose of an aerosol can that isn't empty, discharge the contents of the container into a deep cardboard box outdoors, and allow it to dry. When the can is empty, it and the cardboard box can be thrown in the trash. If you discharge the contents be very careful: Do not spray near children, animals, or areas of human contact such as playgrounds or gardens. Avoid inhaling the vapors. Alternatives: For the most part, aerosol sprays are no more effective than pouring, wiping, brushing, or dusting. Try to purchase products in pump spray, roll-on, liquid, or non-aerosol spray. Spray guns may be desirable in a case where you want to cover a large surface evenly. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects: CHLOROFLUOROCARBONS - When released into the air, destroys the ozone layer in the earth's upper atmosphere. No longer used in aerosol products manufactured in the USA.
AIR FRESHENERSAir fresheners work in one of the following four ways: by interfering with your ability to smell by way of a nerve-deadening agent; by coating your nasal passages with an undetectable oil film; by covering up one smell with another; and (rarely) by breaking down the offensive odor. Despite their name, air fresheners do little to freshen the air. Aerosol fresheners can be harmful to lungs if inhaled in high concentrations or for prolonged periods of time. Solid fresheners may be poisonous if eaten by children or pets. Use: If freshener is in aerosol form, do not breathe fumes. Avoid skin contact. Use only in well-ventilated areas. Storage: Keep out of the reach of children and pets. Store away from heat or flame. Disposal: It is best to use up air freshener as it was intended. For unwanted portions of solid air freshener, allow to evaporate by exposing it to the air. Alternatives: There are several non-toxic ways to freshen the air in your home.
HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and possible effects:
Formaldehyde - A suspected carcinogen and a strong irritant to the eyes, throat, skin and lungs
ALKALIES and ALKALINESAlkalies are commonly found in bleach, Ammonia automatic dishwashing detergent, low phosphate detergents, drain cleaners, oven cleaners, lime, color wave hair preparations, depilatories, alkaline disk batteries, Clinitest tablets for home glucose testing, and wet cement. Alkalies, also called bases, all have a pH range of 7.1 to 14.0. The corrosive effects of alkaline chemicals usually occur rapidly, sometimes with exposures as short as one second. Severe skin irritation and burns can occur from skin contact. Inhalation of fumes from alkalies may cause watering of the eyes, sneezing, coughing, choking, shortness of breath, and inflammation and irritation from the nose to lungs. When working with household products that are alkaline or contain alkalies, wear gloves to protect your skin. Make sure ventilation is adequate. For disposal recommendations, see the specific product such as drain cleaner.
ALL PURPOSE CLEANERSThe ingredients in all-purpose cleaners are a combination of detergents, grease cutting agents, and possibly solvents and disinfectants. These products may contain one or more of the following hazardous ingredients: Ammonia, ethylene glycol monobutyl acetate, sodium hypochlorite, and trisodium phosphate. Depending upon the ingredients contained in the particular cleaner, they can be mildly to extremely irritating to the skin, eyes, nose, and throat, and corrosive if swallowed. Chronic irritation may occur from repeated use. Do not mix ammonia-based cleaners with bleach-based cleaners. Hazardous fumes will result! Cleaners that contain phosphates present a water pollution hazard. Use: Wear gloves. Make sure that the ventilation is adequate. Do not mix different cleaners together as toxic fumes may result. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
AMMONIA - Fumes irritate eyes and lungs; can cause burns or rashes on skin; can produce deadly chloramine gas if mixed with chlorine containing products
ALUMINUM CLEANERSMany aluminum cleaners contain hydrofluoric acid which is extremely corrosive and toxic. Hydrofluoric acid is extremely dangerous! Upon contact, it destroys the flesh down to the bone as the fluoride ion continues to act until it is neutralized by a calcium store. The pain from burns may be delayed for several minutes to several hours, depending upon concentration. During this time, the acid in the aluminum cleaner can burn deeply into the tissue, causing severe burns and possible damage to muscles, ligaments, and bone. Low concentrations in the eyes can cause intense irritation; high concentrations, immediate blindness. I can attest to the extreme dangers of Hydrofluoric Acid because we use it on a daily basis in our Inorganic labs. Hydrofluoric Acid and Fluoboric or Fluoroboric Acid are some of the few acids which will dissolve the silica and alumina based chemical catalysts we analyze. We take special precautions when using Hydrofluoric Acid because unlike the other acids we use, if enough Hydrofluoric Acid contacts the skin it can be deadly! Many have died from relatively small skin surface area exposure to Hydrofluoric Acid. It's an extremely painful way to die! Throughout our labs we have easily accessible vials of Calcium Gluconate which is a cream to be immediately applied upon any skin contact with Hydrofluoric Acid. Calcium Gluconate quenches the reaction of Hydrofluoric Acid with the body's calcium. It's an essential item to have around when using Hydrofluoric Acid because if exposure to Hydrofluoric Acid occurs a pernicious chain reaction ensues which affects tissue and blood eventually resulting in severe damage and likely death. Because our labs use this deadly acid on a daily basis we have notified the authorities so they can be prepared in the event of an emergency due to Hydrofluoric Acid exposure. The local emergency response teams, our own first responder emergency management teams, and the local hospitals have all been equipped with special injectable antidotes and the Calcium Gluconate cream which counteract the effects of Hydrofluoric Acid exposure. STAY AWAY FROM HYDROFLUORIC ACID FOLKS!!! IT'S A KILLER!!! Use: Do not use products with hydrofluoric acid. If the aluminum cleaner ingredients are not on the label, you cannot assume hydrofluoric acid is not in the product. If you are using a product which contains this ingredient, protect all exposed skin in addition to wearing protective gloves, safety goggles, and a respirator with an acid gas cartridge. Storage: Store away from children. Disposal: If aluminum cleaner is in liquid form take it to a household hazardous waste collection. If collection is not available, then flush down the drain with plenty of water. If you are on a septic tank or lagoon, dispose of small quantities over a number of days. If cleaner is in solid paste form and has completely hardened, it may then be thrown in the trash destined for the landfill.
AMMONIAAmmonia, a colorless gas or liquid with a sharp irritating odor, can be found in household cleaners, wax removers, glass and window cleaners, and oven cleaners. In strong concentrations, such as may be found in commercial products, ammonia vapors and liquids can be corrosive causing severe burns and irritation to the skin, eyes, and lungs. Household ammonia contains 5-10% ammonia and is considered to be an irritant rather than a corrosive hazard. Vapors, even in low concentrations, can cause severe eye, lung, and skin irritation. Chronic irritation may occur if ammonia is used over long periods of time. Do not mix ammonia with chlorine bleach or bleach products! When ammonia and bleach are mixed, a chloramine gas results which can cause coughing, loss of voice, feeling of burning and suffocation, and even death. Ammonia inhalers are sometimes mistaken by children for candy. These inhalers or smelling salts will cause burns to the lips and mouth if chewed. Use: Wear protective gloves, safety goggles, and a respirator with an ammonia cartridge. Use ammonia only in well-ventilated areas where there is plenty of fresh air. Storage: Store away from children. Disposal: Empty containers can be thrown in the trash. It is best to use up the product as intended, but if you must dispose of an unused portion, flush down the drain with plenty of water. If you are on a septic tank or lagoon, dispose of small quantities over a number of days. Alternatives: Vinegar, like ammonia, will cut through grease and grime but without the irritation produced by ammonia and ammonia vapors. For much more specific information about ammonia sources, ammonia toxicology, ammonia MSDS, ammonia FAQs, and public health information please visit our Extensive Guide to Ammonia.
AMMUNITIONThe primary danger associated with ammunition is accidental discharge, especially when children of any age view ammunition as something to play with. For example, pounding on a bullet with a hammer to break it open and see what is inside or throwing ammunition into a fire can lead to accidental discharge. Use: Treat weapons with respect. Be certain that the ammunition used is the proper size for the firearm. Storage: Keep away from children. Store in a cool, dry place away from heat or flame. Disposal: Call your local Fire Department or Sheriff's office. They may be able to collect and properly dispose of your unwanted ammunition and fireworks.
ANTI-BACTERIAL CLEANER What is it? Antibacterial cleaners come in a spray can or pump bottle container. They are commonly used in the kitchen to clean things that come in contact with food, like cutting boards and counter tops. Keeping these areas clean will help prevent harmful bacteria from contaminating your food. It is especially important to clean areas that come in contact with raw meats. Raw meats can also carry bacteria. Use an antibacterial kitchen cleaner or wash the area with hot soapy water. If cleaning the kitchen you may be using antibacterial cleaners to do the job. If so, you need to be sure to always read the label first to know how to properly use these products and for safety information.
What's in it?
What health and safety things do you need to think about with antibacterial cleaners?
ANTIFREEZEAntifreeze contains ethylene glycol which is poisonous when ingested. Ingestion may result in depression followed by respiratory and cardiac failure, kidney damage and brain damage. Manufacturers of antifreeze are required to clearly post dangers on the label and provide a child-proof cap, which minimizes the danger of accidental ingestion by children. However, antifreeze when improperly disposed of can endanger the health of pets. Each year, thousands of dogs and cats are poisoned by discarded or leaking antifreeze. The sweet taste of antifreeze attracts pets who lap up puddles of antifreeze they find. To prevent this danger, wash down or absorb puddles of antifreeze with an absorbent material such as kitty litter and dispose of the absorbent in the trash. Use: Follow label directions. Never heat antifreeze. This would release toxic fumes. Storage: Store away from heat and in a well-ventilated area. Keep away from children and pets. Disposal: The major components of antifreeze can be broken down by organisms in a sewage treatment plant. If your home is connected to a sanitary or municipal sewer system, household quantities of antifreeze can be flushed down the drain with plenty of water. The solution is not so easy for those homes with a septic tank because antifreeze can overwhelm the organisms in your septic system, causing damage to the system. If your wastewater goes into a septic tank, very small amounts over a period of time can be flushed with plenty of water. Better yet, ask a friend, relative, or neighbor who is hooked up to the sanitary sewer system to use their drain to dispose of your household quantity of used antifreeze. Do not pour antifreeze into storm sewer openings, sinkholes, or abandoned wells where they will directly pollute the water. HAZARDOUS CONSTITUENT and Possible Effects: ETHYLENE GLYCOL - Poisons animals, who are attracted to the sweet smell; can cause damage to internal organs through skin absorption; inhalation can cause dizziness. There is a new type of antifreeze available that contains PROPYLENE GLYCOL. Propylene glycol is much less toxic than ethylene glycol. An animal would have to consume a lot more of this type of antifreeze, a quantity that is unlikely to be available, to get sick or to die. The bottle's label should tell you what is type of antifreeze it is. Some people who have vacation homes that they "close up" for the winter will pour antifreeze into toilets so the water doesn't freeze. In this case, these people should always use the less toxic antifreeze (the ones with propylene glycol in it) because pets can drink out of toilets and can become poisoned.
ARSENICArsenic is a highly toxic, naturally occurring grayish-white element used as a poison in pesticides and herbicides. Arsenic is also found as an ingredient in pigments and wood preservatives. Arsenic contained in wolmanized lumber will not release toxic compounds unless burned. Some treated lumber contains Arsenic in the form of Copper Chromated Arsenate. Arsenic can be harmful through inhalation, absorption through skin and mucous membranes, skin contact, and ingestion. Accidental poisoning can occur through breathing fumes, licking paintbrushes to a point when using pigments containing arsenic, or from wearing inadequate clothing when applying arsenic-based products. Effects of mild poisoning from inhalation include loss of appetite, nausea, and diarrhea. Effects of more severe chronic or acute exposure include skin lesions, skin rash, chronic headaches, apathy, garlic odor on breath, a metallic taste in the mouth, a bronzing pigment of the skin resembling raindrops on a dusty road," and possible damage to the liver. Arsenic and arsenic compounds are known cancer-causing agents and have been implicated in lung and skin cancer and associated with birth defects. For much more specific information about Arsenic sources, Arsenic toxicology, Arsenic MSDS, Arsenic FAQs, and public health information please visit our Extensive Guide to Arsenic.
ARTS & CRAFTSThe "Labeling of Hazardous Art Materials Act" of 1988 required that any art and craft materials that present a chronic hazard bear a WARNING statement of the hazard, and an additional warning that it is inappropriate for use by children. The Law directed the Consumer Product Safety Commission to set guidelines determining whether arts and crafts present chronic long-term hazards to both adults and children. All arts and crafts materials must identify the hazardous ingredients, provide guidelines for safe use, identify that the product complies with Federal law, and provide a telephone number for the consumer to request additional information. This information must appear on the label, the packaging, or the display for the product. Although this law has been in effect for nearly ten years, there are still products on the market, especially imported art products, which are not in compliance. Permanent felt-tip markers, rubber cement, spray fixatives, powdered clay, and instant papier-mache are standard arts and crafts supplies found in many homes. All of these materials contain chemicals that are hazardous if inhaled, absorbed, or swallowed. Children are especially prone to mishandling, chewing, sucking, inhaling, or swallowing art materials and decorating their hands and faces with them. The Arts & Crafts Materials Institute has successfully sponsored a certification program, certifying that products are non-toxic and meet quality and performance standards. Products in their certification program which have earned the CP (certified product) or AP (approved product) seal include crayons, water colors, tempera colors, finger paints, chalks, modeling materials, block printing inks and media, drawing inks and media, etching inks and media, screen printing inks and media, school pastes and adhesives, acrylic and oil paints and media, marking crayons, and other art materials. Products bearing the AP seal are non-toxic even if ingested. Those bearing the CP seal are non-toxic even if ingested and meet or exceed specific quality standards of material, workmanship, working qualities, and color. Products without these seals but which state they are "non-toxic" indicate only that the product is not acutely toxic and may still make a person sick if swallowed. An excellent source on toxic arts and crafts information is a data sheet entitled "Children's Art Supplies Can Be Toxic," published by the Center for Occupational Hazards. To obtain a copy, send a self-addressed, stamped envelope with your request to 5 Beekman Street, New York, NY 10038. Use: Carefully read labels to identify products which are certified and approved by the Arts and Crafts Materials Institute. A list of these products can be obtained from the Institute, free of charge, by sending a self addressed, stamped envelope and request to 715 Boylston Street, Boston, MA 02116. Refrain from eating or drinking while using these products and wash your hands thoroughly when finished. Alternatives: In order to choose safe art supplies to keep at home, for school projects, or just for fun, consider the following tips:
AVOID... powdered tempera paints, pastels, chalks, or dry markers that create dust.
AVOID... instant paper-mache (may contain asbestos fibers and Lead from pigments in colored printing inks)
AVOID... aerosol sprays
AVOID... oil-based paintss, turpentine, Benzene, Toluene, and rubber cement and its thinner
ASBESTOS
Goto our Complete Guide to Asbestos for complete information about Asbestos including extensive MSDS information, list of specific product brands with Asbestos, jobs, symptoms, and case studies of asbestos exposure. Asbestos is a naturally occurring group of minerals that are flexible, fire resistant, and virtually indestructible. Many hundreds of products contain asbestos fibers. Some general categories are insulation, asbestos cements, fire-proofing, fireproof clothing, floor tiles, pipes, brake and clutch linings, pot holders, ironing board pads, hair dryers, and textured paint. In the recent past, some uses have been banned: spraying asbestos-containing materials (1973); certain pipe coverings (1975); some patching compounds and artificial fireplace logs (1977); spray-on asbestos decorations (1978); and hair dryers containing asbestos (1979). In most products, asbestos is combined with a binding material. However, if the tiny asbestos fibers do become airborne and inhaled, they can remain in the lungs and may cause severe health problems that do not appear until many years later. There is no known safe exposure level to asbestos. Asbestos toxicity surfaces only after a long latent period. The respiratory tract is the usual target organ. Asbestosis and asbestos-related cancers such as Mesothelioma are the two main categories of asbestos disease. Do not dust, sweep, or vacuum particles suspected of containing asbestos. This will disturb tiny asbestos fibers, causing them to become airborne and easily inhaled. Products containing asbestos are not often labeled as such. For information on whether a product contains asbestos, contact the manufacturer, ask people who have worked with asbestos (such as asbestos handlers, plumbers, building contractors, or heating contractors), or contact the United States Consumer Product Safety Commission (CPSC, 1-800-638-2772). Any material containing asbestos should not be disturbed unless necessary. If you think a product contains asbestos and you must disturb it, find a contractor trained in safe procedures for handling asbestos. For further information concerning asbestos, contact the CPSC, American Lung Association or your local office of the State Department of Health. Vermiculite insulation mined in Libby Montana in the 1990s and known by the brand name Zonolite has also been proven to contain dangerous levels of Asbestos contamination and may have been placed in millions of U.S. home attics! An indictment has been brought against the manufacturer, W. R. Grace and Company, and the whole vermiculite insulation fiasco has been called the worst case of widespread public exposure to a hazardous material in history! For all the details, pictures, and resources about the public health crisis, visit our guide to vermiculite insulation. In some cases homes have been completely contaminated with Asbestos fibers resulting from the Asbestos tainted Vermiculite insulation.
ASPHALT / ROOFING TARIn the paving and roofing trades, a tar or asphalt is applied in a hot liquid form that cools into a semi-solid covering. Asphalt is a residue of petroleum refining. Tar is produced by distillation of coal, oil, lignite, peat, or wood. Inhalation of hot asphalt fumes can cause eye and respiratory tract irritation, headaches, nausea, and nervousness. Skin exposure to hot tar can cause serious burns. Wear protective gloves.
AUTOMATIC TRANSMISSION FLUIDAutomatic transmission fluid, used to pull the clutch and lubricate automobile transmissions, is mainly composed of mineral oil. Automatic transmission fluid is flammable at high temperatures and relatively non-toxic unless swallowed and aspirated (sucked into lungs during swallowing or vomiting). Used automatic transmission oil contains environmentally toxic heavy metals including Lead. The heavy metal in used fluid can cause severe nervous system damage to wildlife and other animals if disposed of improperly. Use: When draining fluid wear gloves and avoid skin contact. Storage: Store used transmission fluid in a plastic container with a tight-fitting lid. Clearly mark what is in the container and store on a high shelf out of the reach of children and pets. Disposal: If not contaminated with other products, used and unused automatic transmission fluid may be accepted for recycling at local service stations that also accept used motor oil or at the highway transportation department. Ask first before dumping the used fluid into an oil collection tank because some centers may not accept it. Carry the transmission fluid in a plastic container with a tight-fitting lid or, if the fluid is unused, in its original container. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects: GLYCOLS - Some compounds cause kidney damage
BATTERIES - DRY CELLDry cell and disc or button batteries are used in flash lights, radios, hearing aids, watches, cameras, calculators, toys, and other items in the home. These batteries may contain zinc, Lead, alkalines, Mercury, nickel, cadmium, silver, and electrolytes. If batteries leak or explode the chemical substances contained in these batteries can cause internal and external burns and irritation. Batteries which explode can spew their contents on unsuspecting victims. There are two primary reasons that batteries explode: if an attempt is made to recharge non-rechargeable batteries, gases may build up and generate enough pressure to explode the battery; and batteries which are thrown into a fire, burned in a barrel, or otherwise incinerated can explode. Batteries which are chewed on or punctured can also leak. Discarding batteries poses a clear environmental danger. Batteries contain heavy metals, such as silver, nickel, cadmium, lead, mercury, lithium, manganese, and zinc, which can accumulate and concentrate in waterlife, wildlife, and humans. An example of the danger posed by batteries is that one mercury battery contained in six tons of garbage exceeds the allowable limit for mercury in solid waste as established by the federal government. Use: Keep batteries away from children. Do not, under any circumstances, put disc batteries into your mouth. They are slippery and easily swallowed. Storage: Keep away from flames and out of the reach of children. Disposal: Mercury-oxide and silver-oxide button batteries are often collected by jewelers, pharmacies, and hearing aid stores who sell them to companies that reclaim the metals. Many communities across the United States are separating batteries from their waste stream and contracting with companies to provide recycling, neutralization, or proper disposal. Alkaline, carbon-zinc, and lithium batteries do not have a ready market available at present. Some recycling companies have recently started accepting nickel-cadmium batteries and are willing to provide collection containers and pay for bulk transportation to the processor and recycling facilities. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
NICKEL - Causes dermatitis, sensitizer
BATTERIES - WET CELLAutomobile, boats, and tractor batteries are wet cell batteries which contain Lead and a solution of sulfuric acid. When activated, the electrolyte solution in the battery produces explosive gases which are easily ignited. Manufacturers of batteries containing sulfuric acid must use labels which warn consumers of the dangers from battery acid and accumulated gases. Sulfuric acid is extremely caustic. Fumes are strongly irritating, and contact can cause burning and charring of the skin; it is exceedingly dangerous to eyes. Lead is poisonous in all forms and accumulates in our bodies and in the environment. Use: Wear protective gloves. Do not get battery acid on you or your clothing. If you do, wash your hands or body immediately and put baking soda on your clothes where the battery acid splashed. Do not attempt to neutralize acids on the skin or when swallowed. Flushing with or drinking sodium bicarbonate creates thermal heat from the acid base reaction, causing further injury. Do not stand by an uncapped battery while the motor is running; it can splash on you. After touching a battery, wash hands thoroughly before touching eyes or mouth. Keep all sources of flames, including cigarettes, away from batteries. Storage: Store away from children, especially curious children who might want to break open the battery to see what is inside. Keep away from all sources of sparks, including flames. Store under a tarp or in a covered area. Disposal: Recycle used batteries! Improper disposal of batteries presents an environmental hazard. It is important and easy to dispose of batteries by recycling them and it is usually possible to trade in old batteries where you purchase new ones. To locate the recycler nearest to you, look up "Batteries" in the Yellow Pages of the phone book. Depending upon the market place, you may get a small amount of money for your recycled battery, but the fact that you do not have to pay to dispose of this highly hazardous waste makes it a bargain to recycle batteries. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
SULFURIC ACID - Corrosive, causes severe skin burns, and can cause blindness
BENZENEBenzene is a highly flammable, highly toxic, aromatic petroleum distillate product which is a colorless to light yellow liquid with a pleasant odor. In 1978 the Consumer Product Safety Commission banned the use of benzene in many household products. However, it may still be found in some varnishes, paint and varnish removers, airplane glue, nail polish remover, pesticides, and lacquers, and as a solvent for waxes, resins, and oils. It is also used as an anti-knock agent in Gasoline. Visit Our Complete Guide to Benzene for everything you could ever want to know about the hazards of Benzene. Benzene is highly flammable and poisonous when ingested or inhaled. It is irritating to mucous membranes. Avoid skin contact and fumes. Harmful amounts may be absorbed through the skin and may cause sensitivity to light, producing skin rashes and swelling. Inhalation of fumes can be acutely or chronically toxic. Benzene is a carcinogen. For more than a century, scientists have known that benzene is a powerful bone marrow poison, destroying the bone marrow's ability to produce blood cells. Environmental Exposure to organic solvents such as Benzene and other petroleum products have been associated with a higher risk of developing Acute Myeloid Leukemia. Acute Myeloid Leukemia is the most common form of myeloid leukemia in adults (chronic lymphocytic leukemia is the most common form of leukemia in adults overall). In contrast, acute myeloid leukemia is an uncommon variant of leukemia in children. The median age at diagnosis of acute myeloid leukemia is 65 years of age, and approximately 9,000 individuals are affected by Acute Myelogenous Leukemia in the United States annually. Workers exposed to certain chemicals, such as benzene, over a long period of time are at a higher risk for AML. Specific chromosomal aberrations, including the 8:21 translocation associated with AML, have been detected in the white blood cells of benzene workers before the detection of leukemia. Workers in the petroleum industry prior to 1960 appeared to have an increased incidence of AML, but more recent studies have not shown this. This change may represent better and safer working conditions. The incidence of AML is increased in areas of high automobile density, possibly as a result of exposure to benzene from gasoline. This observation is further supported by the fact that no other cancer or leukemia is increased in areas of high automobile density.
BLEACHLiquid household chlorine bleaches contain approximately 5% sodium hypochlorite solution. When properly used, chlorine bleach can be a simple and effective disinfectant. Chlorine bleach liquid and vapors are irritating to the skin, eyes, nose, and throat. Dermatitis may result from direct skin contact. Ingestion can cause esophageal injury, stomach irritation, and prolonged nausea and vomiting. Bleach, when mixed with acidic substances such as Ammonia, toilet bowl cleaners, drain cleaner, or vinegar, forms toxic gases which can cause coughing, loss of voice, a feeling of burning and suffocation, and even death. Do not mix bleach with other cleaners! Use: Wear protective gloves. Use only in well-ventilated areas with plenty of fresh air. Storage: Store in a well-ventilated area and away from children. Disposal: Use up as intended. To dispose of unwanted portions, flush down the drain with plenty of water. If you are on a septic tank or lagoon, dispose of small quantities over a number of days. Alternatives: For household disinfecting, borax is an option. For bleaching clothes, oxygen (dry) bleaches work well. If you are sold on chlorine bleach, reduce the amount of liquid bleach used in your wash. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects: CHLORINE - Fumes highly irritating to eyes and respiratory tract; causes deadly chloramine gas if mixed with ammonia
BRAKE FLUIDHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects: GLYCOLS - Some compounds cause kidney damage
CAMPHORCamphor is a colorless or a white crystal granule or cake product obtained from the wood of the camphor tree. It may also be synthetically derived. Some products such as lotions, astringents, and moth repellents still contain camphor as an active ingredient. In 1980, the Food and Drug Administration set a limit of 11% allowable camphor in consumer products and totally banned products labeled as camphorated oil, camphor oil, camphor liniment, and camphorated liniment. Camphor, readily absorbed through the skin, produces the sensation of warmth and slight local anesthesia. Camphor poisoning produces seizures and may be preceded by mental confusion, irritability, neuromuscular hyperactivity, and jerky movements of the extremities. Camphor poisoning from household products may occur following oral ingestion. Symptoms occur five to ninety minutes following ingestion.
CARBON MONOXIDECarbon monoxide is a colorless gas which is practically odorless, tasteless, and non-irritating. Carbon monoxide is always formed when a fuel containing carbon is inadequately burned with poor ventilation. Kerosene, charcoal, coal, wood stoves, and automobile exhaust fumes are common sources of carbon monoxide poisoning. Natural gas in the United States does not contain carbon monoxide, but it may form if the gas is burned without adequate air supplies. Carbon monoxide starves the body and brain of oxygen. Carbon monoxide poisoning produces symptoms ranging from headache, dizziness, flushed skin, disorientation, troubled thinking, abnormal reflexes, shortness of breath, fainting, and convulsions, to coma and even death. Heart problems are also aggravated by the presence of carbon monoxide because the heart must pump harder. Children, persons with respiratory illness or anemia, and the aged may be particularly sensitive. Chronic exposure to low carbon monoxide levels impairs judgement and increases the time required to make decisions. If you have an attached garage, always make sure the door to the house is closed and the garage door is open when the car is running. If you think that you have a problem with carbon monoxide fumes, contact your local or state Department of Health for assistance.
CARBON TETRACHLORIDEBecause of its excellent solvent properties and non flammability, carbon tetrachloride has been in use for many decades in commercial products such as dry cleaning solvents, grease solvents, and fire extinguishing agents. Today it is used only in industry and as a fumigant. In 1970, the Food and Drug Administration banned carbon tetrachloride and any mixture containing it for use in the home. The FDA classified carbon tetrachloride as a substance so hazardous that no warning label could be devised that would adequately protect the householder. Carbon tetrachloride is a cellular toxin that produces cellular destruction throughout the body, especially in the liver, kidney, and central nervous system. It is toxic by all routes of exposure: inhalation, absorption, skin contact, and oral ingestion. Although uniquely potent, carbon tetrachloride is in many respects representative of a large class of related chlorinated hydrocarbon solvents. Disposal: If you find a product containing carbon tetrachloride, secure and hold for professional household hazardous waste collection or give it to a licensed hazardous waste handler.
Carburetor CleanerHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
CRESOL - Corrosive to tissue, damages liver, kidneys, lungs, pancreas and spleen
CARPET CLEANERHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
PERCHLOROETHYLENE - Fumes are carcinogenic and acutely toxic, cause dizziness, sleepiness, nausea, loss of appetite and disorientation These ingredients are most commonly found in commercial "spot removers", rather than water-based detergent products or rub-in cleansing powders.
CAR WAXPaste wax typically contains 75-85% petroleum naphtha and 15-25% wax. Naphtha is flammable and an irritant that can enter your system through inhalation, ingestion, and skin and eye contact. Skin chapping and sensitivity to light may develop with repeated and prolonged contact. Use: Wear protective gloves. Storage: Make sure wax is tightly capped. Keep out of reach of children. Disposal: It is best to use up car wax as intended to avoid a waste problem. If the car wax has hardened, it can be disposed of in the trash destined for the landfill.
ChlorofluorocarbonsChlorofluorocarbons (CFCs) are a class of man-made chemicals known by such tradenames as "Freon," "Genetron," and "Isotron." CFCs have been used in a wide variety of manufacturing steps and products including as a solvent in the electronics industry, foaming or blowing agent, aerosol propellant, fire extinguisher agent, dry cleaning solvent, degreasing agent, a key component in making rigid foam insulation for houses and household appliances, and foam packaging insulation material (known by the trade name of "Styrofoam"). Use of CFCs has declined as concern over their interaction with the environment has grown. Inhalation of high concentrations affects the nervous and respiratory system. Initial symptoms include a reduced ability to concentrate, dizziness, headaches, and bronchial constriction, which may lead to sudden death. Intentionally inhaling CFCs from aerosol cans has resulted in the deaths of several teenagers. Chlorofluorocarbons are highly volatile compounds, contributing to air pollution. CFCs are unusual because they do not break down when vaporized into the atmosphere. Instead, they rise slowly through the atmosphere, taking six to eight years to reach the stratosphere (the highest layer of the earth's atmosphere). Here CFCs can reside for more than 100 years. If global CFC production was stopped today, we would still experience the effects for over one hundred years. Chlorofluorocarbons are implicated in two major threats to the global environment: the greenhouse effect and the reduction of the ozone layer. CFCs ontribute to the greenhouse effect, warming the atmosphere by trapping heat which is then radiated back into the atmosphere. CFCs are more than 10,000 times as effective at trapping this radiated heat than carbon dioxide. CFCs have also been shown to contribute to the depletion of the protective ozone layer in the atmosphere. In 1984 the ozone hole formed in Antarctica was larger than the United States and taller than Mount Everest. Ozone levels are depleted most dramatically in the Antarctic, but are being reduced around the world. As the chlorofluorocarbons break down, they release a chlorine atom which is capable of destroying tens of thousands of ozone molecules before being washed out of the atmosphere. Depletion of the ozone layer permits greater amounts of ultraviolet radiation to reach the earth. The increase in ultraviolet radiation affects human health by increasing the likelihood of developing skin cancer and cataracts and may depress the human immune system. Increased ultraviolet radiation reduces crop yields, depletes marine fisheries, damages construction materials, and increases smog. Between 1969and 1986, the average global concentration of ozone in the stratosphere had fallen about 2%. World-wide recognition of the global threat from CFCs has begun. In 1977, the EPA and FDA banned the use of CFCs in the production of most aerosol cans in the United States. However, these chemicals need to be reduced on a global scale. In 1987, thirty-five countries signed the Montreal Protocol on Substances that Deplete the Ozone Layer. Provisions of the agreement include a freeze on CFC production at 1986 levels by 1989, a 20% reduction by 1993, and a 50% reduction of 1986 levels by 1998. However, governments which signed the Protocol need to enforce compliance and nations that did not sign it must agree to reduce CFC production for these measures to be effective. This is a global problem which we all can help solve at home. As consumers, we can influence industrial and government decision-makers with our dollars and votes. Do not use aerosol products. Avoid purchasing products wrapped in foam packaging material (In 1989, McDonalds has been involved in a campaign to recycle styrofoam containers of all kinds. You may wish to check with your local McDonalds to see if they are willing to accept styrofoam containers.) Check your air conditioning (in home and car) and refrigeration systems for leaks and have them sealed. When having the air conditioning system of your car recharged, patronize service stations which clean and recycle used coolant, rather than vaporizing it into the atmosphere. Use materials other than rigid foam insulation (blown in with CFC-1 1 or CFC-1 2) to insulate your home. Support legislation for reducing the amount of OFOs produced and for compliance with the Montreal Protocol.
CRESOLCresol, a highly caustic, colorless solid or liquid with a sweet tarry odor, is used mainly as a disinfectant. Cresol is very corrosive to all tissues. When it comes in contact with the skin it may not produce any burning sensation immediately. Prickling and intense burning will occur followed by loss of feeling. If cresol contacts the eyes it may cause extensive damage. Cresol vapors and liquids are absorbed through inhalation and eye and skin contact. Repeated or prolonged exposure to low concentrations of cresol can produce chronic systemic poisoning. Symptoms of poisoning include vomiting, difficulty in swallowing, diarrhea, loss of appetite, headache, fainting, dizziness, mental disturbance and skin rash. Cresol attacks the central nervous system, respiratory system, liver, kidneys, skin and eyes.
DETERGENTThe word "detergent" refers to household cleaning products which are based on non-soap, synthetic surfactants and which are primarily used for laundering and dishwashing. There are several types of detergents including automatic dishwashing, hand dishwashing, enzyme, and low-phosphate detergents. All detergents contain "cationic," "anionic," or "non-ionic" detergents. Cationic detergents are the most toxic when taken internally. Symptoms from ingestion include nausea, vomiting, shock, convulsions, and coma as quickly as one to four hours after ingestion, due to rapid absorption. By themselves, anionic detergents have low toxicity causing mild, local irritation of skin and eyes. But the addition of "builders" to anionic detergents is common and makes anionic detergents alkaline and caustic. Non-ionic detergents have low toxicity. At most, mild irritation of the skin and mucous membranes occurs. Ingestion causes no hazardous effects. Some typical nonionic detergents are alkyl aryl polyether sulfates, alcohol sulfonates, alkyl phenol polyglycol Ethers, and polyethylene glycol alkyl aryl ethers. Detergents are responsible for many household poisonings. Part of the problem is that detergent boxes are brightly colored and attractive and commonly stored in low, accessible places. There is a common misconception that low-phosphate detergents are "safe." While low phosphate detergents are safer to the environment, they are 100 to 1000 times more caustic than phosphate detergents. This means that low-phosphate detergents can cause serious burns if even a small amount is ingested. Since powdered granules are more difficult to accidentally swallow, powdered rather than liquid detergents may be a safer choice if you have small children in the home. All detergents should be carefully stored well away from the reach of children. - Automatic Dishwashing Detergent - Most automatic dishwashing detergents are alkaline with pH values of 10.5 to 12.0. These products may be classified as irritants or corrosives depending upon their composition, concentration, and physical form. Skin irritation or burns may occur following exposure to dissolved detergents. Toxicity may range from mild tissue causes severe burns. The fact that automatic dishwashing detergents contain phosphate causes environmental concerns. You might consider buying a powdered automatic detergent over a liquid variety, because powdered detergents are more difficult to mistakenly swallow Automatic dishwashing detergents may also contain sodium carbonate. - Hand Dishwashing Detergent - These products are intended for the handwashing of dishes. Hand dishwashing detergents are much less toxic than automatic dishwashing detergents. Hand dishwashing detergents are combinations of anionic and non-ionic detergents, glycols, alcohols, and salts. Exposure to the membranes of the mouth, throat, and gastro-intestinal tract may be irritating but not caustic. Anionic and non-ionic detergents are not well absorbed, and no toxic dose has been established. Hand dishwashing detergents are generally considered low in toxicity. - Enzyme Detergent - Enzymes are found in various laundry detergents and pre-soaks to loosen soil and remove stains. The enzymes are obtained from selected strains of bacteria. Products which contain enzymes have irritating and sensitizing properties. Asthma and dermatitis may occur from industrial exposure to these enzyme products but would be unlikely from routine household use. Granulated detergents, which encapsulate the enzyme, are less toxic than powdered formulations to people who have become sensitized to these enzyme detergents.
DIESEL FUEL & KeroseneBoth kerosene and diesel fuel are flammable and are petroleum distillate products. Kerosene is used in lamps, domestic heaters or furnaces, jet engine fuel, and as a solvent for greases and pesticides. Diesel fuel has a higher boiling point than kerosene and is used to power diesel engines. Kerosene and diesel fuel can damage your health through inhalation, ingestion, and skin contact and absorption. The first symptoms of poisoning include confusion, restlessness, and tremors. Overexposure can lead to central nervous depression with symptoms of inebriation. This may be followed by nausea and headache and may eventually lead to coma and death. Aspiration of fluid into the lungs can occur during ingestion and vomiting. This may result in chemical pneumonia and lung lesions. Ingestion of kerosene is a special problem since it is frequently improperly stored in food containers (such as soda pop bottles) and then swallowed by children. Use: Never smoke around kerosene or diesel fuel. Keep the lid on when not in use. Do not use kerosene or diesel fuel to clean paint or grease from your body (use detergent and water instead or massage with a few drops of baby oil, butter or margarine, wipe dry, and wash with soap and water). Always wear protective gloves and wash your hands and exposed body parts before eating or smoking. Avoid breathing fumes. If using a kerosene heater, provide adequate ventilation to remove combustion pollutants, such as carbon monoxide and sulfur dioxide. Use only low sulfur 1-K grade fuel in kerosene space heaters. Never use home heating oil or other fuels. Storage: Keep out of the reach of children and pets. Store in an approved safety container in a garage or outbuilding with good ventilation. If you have a water heater, furnace, or other sources of ignition in your garage, it may not be a safe place to store kerosene or diesel fuel. Keep away from heat, flame, and sources of ignition. Do not completely fill the container; kerosene and diesel fuel need room to expand. Disposal: There is usually little need to dispose of kerosene or diesel fuel since it can normally be used. However, kerosene or diesel fuel that has been contaminated or dirtied cannot be used and must be saved for disposal by a licensed hazardous waste collector or through a professional household hazardous waste collection program.
DISINFECTANTSDisinfectants are considered pesticides. They reduce some germs and are a temporary measure at best for making your home "germ free." Skin contact and vapors can be irritating and corrosive to the respiratory system and skin. Disinfectants are especially hazardous when dispersed from aerosol cans because the disinfectant can be easily ingested through the nose and mouth. Disinfectants may contain one or more of the following hazardous substances: Ammonia, cationic detergents, cresol, lye, phenol, pine oil. Please refer to these compounds for specific health hazards associated with these ingredients. Use: Avoid aerosol dispensers. Handle disinfectant with gloves to avoid corrosive effects and absorption through skin and wear safety goggles. Make sure ventilation is adequate with plenty of fresh air present. Do not use disinfectants around food, animals, or children. Storage: Keep away from children. Store in a well ventilated area. Disposal: Use up as intended. To dispose of unused or unwanted portions take the product to a hazardous household waste collection center. If collection is not available, then flush the product down the drain with plenty of water. If on a septic tank or lagoon, dispose of small quantities over a number of days. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
Ammonia - Fumes irritate eyes and lungs; can cause burns or rashes on skin; can produce deadly chloramine gas if mixed with chlorine containing products
DRAIN CLEANERSChemical drain cleaners (also called drain openers) are extremely corrosive and dangerous to use. Common ingredients in drain cleaners include lye or sulfuric acid. These chemicals work by eating away materials, including your skin if it should come in contact. Likewise, vapors are harmful. If you are on a septic system, you should know that drain cleaners are hard on your system as they kill the microbial bacteria which are necessary to the workings of your septic tank. The use of chemical drain cleaners as a "preventative" measure is not a good idea. Boiling water or a handful of baking soda and half cup of vinegar poured down the drain weekly is at least as effective as a chemical drain cleaner and much, much safer for you and the environment. Also effective, particularly in preventing clogs, are many brands of enzymatic cleaners. If you have used a chemical drain cleaner and the clog still exists, Do not try to clear the drain with a plunger or pressurized drain opener. This would only invite splashback. Also, do not add other cleaners to the drain following the use of a commercial drain cleaner. The combination of chemicals can produce toxic gas or become reactive and blow out of the sink and on to you. If a chemical drain cleaner has done nothing to help your clog and you still have standing water, then there is no reasonable choice except to call a professional to fix the clogged and now contaminated drain. Be sure to tell them what product was used in the drain so that they may adequately protect themselves. If a drain cleaner claims to be "non-caustic" or "noncorrosive," it should state its ingredients. The product may still be poisonous if inhaled in heavy concentrations or swallowed. Use: Wear protective gloves and safety goggles. Avoid fumes. Storage: Store away from children. Disposal: Use up as intended. Take unused product to a hazardous household waste collection center. If collection is not available and if you are connected to a sanitary sewer or municipal sewer treatment, you may dispose of unwanted portions of drain cleaner by flushing down the drain with plenty of water. If you are on a septic tank or lagoon, small amounts of drain cleaner may be flushed with plenty of water over a number of days. It would be best, however, to ask a friend, relative, or neighbor who is on a sanitary or municipal system to allow you to use their drain to dispose of your household quantity of drain cleaner. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
LYE - Caustic causing burns to skin and in severe cases, blindness
DRY CLEANINGCarbon tetrachloride, now banned from household products, was the favorite solvent cleaner used in these products. A leading substitute, perchloroethylene or PERC, is a volatile, nonflammable solvent, that is fatal in large doses. There is concern over the chronic inhalation of perchloroethylene. The primary effect from acute and chronic inhalation of vapors is depression of the central nervous system. Other toxic chemicals often found in spot removing products include trichloroethane, ethylene dichloride, naphtha, Benzene, and Toluene. For specific health effects of these ingredients please refer to the individual headings. All of these solvents present an inhalation and ingestion hazard. Some also present a hazard through skin absorption. Use: Wear nitrile gloves and arrange your work so that the fumes are blowing away from you. Do not allow children or pets into the room where you are working. Keep the lid on the fluid product as much as possible to avoid the solvent from volatilizing and being breathed. If you spill spot remover or dry-cleaning fluid on your skin, wash it off immediately with soap and water. If the solvent spills and puddles, absorb it with kitty litter and throw the wet absorbent material in a trash can outdoors. Never use dry-cleaning fluid or spot remover in a washing machine or put articles that are damp with solvent in a dryer. When you bring clothes home that have been dry-cleaned, take the plastic bag off and allow the clothes to air out well before wearing. When using a dry cleaning machine, to reduce vapors allow the door to remain ajar for a few minutes after the operation is complete. The solvent will evaporate quickly. Remove garments from the machine and allow to cool before handling. Storage: Store away from heat and flames in a box lined with plastic bags. Disposal: Currently available means offer no good way to dispose of leftover dry-cleaning fluid or spot remover. These solvents should be disposed of by a licensed hazardous waste handler or saved for a professional household hazardous waste collection. The best way to eliminate a waste problem is to carefully use up these products as they were intended. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
CARBON TETRACHLORIDE - Destructive to liver, kidney, and central nervous system by inhalation, absorption, skin contact, or ingestion
DYESThere are many types of dyes and which one you use depends primarily on the type of fabric that is to be dyed. Some of these dye types are known to be toxic or corrosive, some cause allergies (sensitizing), and some have long-term (chronic) health effects such as cancer. The chronic effects of most synthetic and natural dyes have not been fully researched. Many of the chemicals used in dyeing fabrics are hazardous to your health through skin contact and inhalation. Many dyes require additional chemicals (mordants) to bind the dye into the cloth fibers. Mordants are used with both natural and synthetic dyes and can cause serious health effects. Some mordants are Ammonia (a skin irritant), oxalic acid (a corrosive), and potassium dichromate (toxic when inhaled). Two common types of dyes used in the home are natural and direct dyes. Natural dyes (also known as mordant dyes) require the use of a mordant and are prepared from plants, insects, and algae. These dyes are used to color cotton and silk. Since most natural dyes are prepared by soaking the natural product, there is no hazard due to inhalation. However, since the mordant may be hazardous, be sure to protect yourself from skin absorption. All commonly available household dyes are direct dyes. These dyes are used for coloring cotton, linen, or viscose rayon. Table salt is used as a dyeing assistant and the dyes require heat in order to set. Many direct dyes are based on benzidine or benzidine derivatives, which are highly toxic by inhalation and ingestion, and possibly through skin absorption. Benzidine and its derivatives are known carcinogens. To find out more about dyes, request the data sheet "Dyes Hazards and Precautions" published by the Center for Occupational Hazards. To obtain a copy, send a self-addressed, stamped envelope with your request to: 5 Beekman Street, New York, New York 10038. Use: If you use fabric dyes, please follow these general rules for safe use.
Storage: Store materials in break-resistant containers. Label all containers clearly and cover them tightly. Store out of reach of children and pets. Disposal: Use up unmixed dyes as directed or share dyes with others who will us them. Contact your local waste water treatment facility concerning the disposal of mixed and unmixed dyes.
ENGINE DEGREASERHAZARDOUS CONSTITUENT and Possible Effects: CHLORINATED solvents - Central nervous system depressants, irritants, vary in toxicity
FERTILIZERFertilizers are plant food supplements which commonly contain nitrogen, phosphorus, and potassium. The numbers on the fertilizer bag (e.g. 10-8-6) refer to the percentages by weight of nitrogen, phosphorus, and potassium, respectively. In general, liquid and granular fertilizers used for house plants and in the garden have a low degree of toxicity unless ingested in large quantities. Single ingredient fertilizers such as ammonium nitrate or lime are more likely to be toxic or corrosive. Environmentally, overuse of fertilizers has resulted in contamination of surface water and groundwater. Excess nitrogen in drinking water (above 10 parts per million nitrate-nitrogen) can lead to methemoglobinemia (oxygen starvation or blue baby syndrome), especially in children under the age of one, elderly persons, and sensitive farm animals such as hogs. Excess phosphorus in the water will result in algae blooms, increased biological oxygen demand, and fish kills. Use: Carefully read the label before purchase and use. Follow all label directions, applying only the recommended amount. Twice as much fertilizer does not work twice as well and only increases the chance of runoff into surrounding water supplies. Wear gloves when handling fertilizer. Storage: Store in a tightly sealed plastic bag away from children and pets. Clearly label the bag with the contents and store away from moisture. Disposal: The best way to eliminate fertilizer waste is to use it up as intended. If you no longer want your fertilizer, check with a relative, neighbor, or friend. Fertilizers are usually in demand in the spring and summer months. If you are unable to find a way to use up your excess fertilizer and it does not contain pesticides, it may be placed in the trash destined for the landfill. If it contains pesticides, follow the procedures under Pesticides. Alternatives: Animal manure, green manure, and compost are time-honored alternatives to synthetic plant fertilizers.
Flea Collars
What is it? Be sure that your pet is not too young to wear a flea collar. They should not be used on young puppies or kittens. Make sure, too, that the collar fits your pet properly. Extra collar length that is sticking out could tempt your pet to chew on it. Or it could get caught in something and possibly cause your pet to choke. Don't tuck the extra length back into the collar either. It could come loose and stick out. You could also be exposing your pet to a higher level of pesticide than is recommended. Always read the label first to find out how to properly use flea collars, how long they last and for safety information.
What's in it?
What health and safety things do you need to think about with flea collars?
FLOOR CLEANERSHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
PINE OIL - Irritates eyes and mucous membranes
FOGGERS (aka Bug Bombs, Total Release Foggers)
What is it?
What's in Foggers?
What health and safety things do you need to think about with foggers or "bombs?" Also, toys should be taken out of the room or put away. No food, plates, cups, silverware or things you cook with should be left out anywhere. The tops of tables and counters should be cleaned before you use them. After the fogger has finished doing it's job the house has to be aired out before people can spend time inside again. Turn on your air conditioner, or open the windows. Using fans will help air out the house faster too. If one of these products is being used in your home, be sure to ask your parents how long you have to stay out of the house or when it will be safe to go back inside. Another important thing to know is that the contents of foggers can be flammable. Because of the way foggers and "bombs" work, they often contain flammable propellants such as IsoButane. The flammable potential of foggers was confirmed during an interesting experiment in one episode of the Discovery Channel's MythBusters with Adam Savage and Jamey Hyneman. A fire or explosion can happen if they are not used according to the directions. Some common symptoms you might get if you come in contact with foggers or "bug bombs" are burning in your eyes and on your skin or trouble breathing. How you are affected and how sick you might get depends on your exposure to the pesticide. This is very important to know.
FORMALDEHYDEFormaldehyde, also known as formalin, formal, and methyl aldehyde, is a colorless liquid or gas with a pungent odor. It is generally known as a disinfectant, germicide, fungicide, defoamer, and preservative. Formaldehyde is found in adhesives, cosmetics, deodorants, detergents, dyes, explosives, fertilizer, fiber board, garden hardware, germicide, fungicide, foam insulation, synthetic lubricants, paint, plastic, rubber, textile, urethane resins, and water softening chemicals. Here is our Complete Guide to Formaldehyde which includes extensive health and MSDS information about the Brands, diseases, symptoms of exposure, sources, and jobs involving Formaldehyde hazards. Inhalation of vapors produces irritation to the eyes, nose, and throat and frequently results in upper respiratory tract irritation, coughing, and bronchitis. Asthma may occur in sensitive individuals. Severe exposure to fumes may lead to chemical pneumonia. Skin reactions after exposure to formaldehyde are very common because the chemical can be both irritating and allergy-causing. In addition, formaldehyde is involved in DNA damage and inhibits its repair. Formaldehyde is a suspected human carcinogen and has been shown to produce mutations and abnormal organisms in bacterial studies. Formaldehyde fumes are liberated from plywood, particle board, and chipboard, as well as urea formaldehyde foam insulation. Symptoms associated with exposure to formaldehyde fumes include mucous membrane irritation, upper respiratory tract irritation, eye irritation, skin rashes, itching, nausea, stuffy nose, headaches, dizziness, and general fatigue. Toxicity is primarily related to the presence of formaldehyde gas. Toxicity may be relatively inconspicuous and nonspecific in nature. Patients suffering from formaldehyde toxicity have been mis-diagnosed as having asthma, bronchitis, anxiety, depression, or hypochondria. Severe prolonged vomiting and diarrhea in infants may be related to chronic exposure to formaldehyde fumes. An individual may become sensitized to formaldehyde following repeated exposure to these fumes. If you have any questions or concerns about formaldehyde levels in your home, contact the office of air pollution control, your local or state Department of Health, or the American Lung Association office nearest you.
FURNITURE CLEANERHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
PETROLEUM DISTILLATES - Irritate skin, eyes, respiratory tract; may cause fatal pulmonary edema; flammable
FURNITURE POLISHThere are three general types of commercial furniture polish: solvents, emulsions, and aerosol sprays. Each type contains specific chemicals which aid in the application of the wax or oil to the furniture surface. Solvent polishes use a chemical solvent to dissolve the oil or wax into a liquid form. Emulsion polishes suspend the oil or wax in a liquid, usually water. Aerosol sprays are solvents or emulsion types packed under pressure. Most polishes are flammable. Furniture polish may contain one or more of the following substances: Ammonia, naphtha, nitrobenzene, petroleum distillates, and phenol. The health dangers most often associated with furniture polish are inhalation of fumes or vapors (especially from aerosols) and poisoning from ingestion. Polishes that look drinkable, like strawberry soda or milk, are especially tempting to children. Use: When using furniture polish you should wear gloves, avoid skin contact with the polish, and provide adequate ventilation. Avoid polishes or stains with nitrobenzene. Storage: Store away from children and sources of flame. Disposal: Unused or unwanted portions of furniture polish which contain petroleum distillates or nitrobenzene should be held for a hazardous waste collection rather than disposing of them in the trash. The best way to avoid a disposal dilemma is to fully and carefully use the product up.
GASOLINEGasoline, a petroleum distillate product combined with various additives, is flammable and highly toxic. Leaded gasoline contains tetraethyl Lead, a highly toxic metal compound. Unleaded gasoline contains high octane components such as Benzene (a known human carcinogen), ethylene dichloride (a known animal carcinogen), and methanol (a highly toxic compound). Gasoline can be harmful to your health through skin contact, skin absorption, inhalation, or ingestion. The first symptoms of poisoning include flushing, slurred speech, staggering, and confusion. Overexposure may result in coma and death. Antioxidants added to keep gasoline from decomposing and forming resins can cause burns to skin and eyes. Use: Never smoke around gasoline. Keep the lid on the can when not in use. Never siphon gasoline using the mouth because chemical pneumonia may result. Do not:
When handling gasoline wear NBR rubber, nitrile, or polyvinyl chloride gloves and thoroughly wash your hands before eating or smoking. Avoid breathing vapors. Storage: Keep out of the reach of children and pets. Store in an approved safety container in a garage or outbuilding with good ventilation. If you have a water heater, furnace, or other source of ignition in your garage, it may not be a safe place to store your gasoline. Keep away from heat, flame, and sources of ignition. Do not completely fill the container - gasoline needs room to expand. While it is a good idea to carry an empty gasoline can in the car, do not keep the can filled with gasoline; the gasoline could explode upon impact. Disposal: Generally, disposal of gasoline is no problem because it will be used up in an engine. However, dirty or contaminated gasoline cannot be burned in engines and must be saved for disposal by a licensed hazardous waste contractor or through a professional household hazardous waste collection program. For this reason, and health reasons, do not use gasoline as a cleaner or solvent. Never mix gasoline with waste oil. This would produce a highly flammable mixture. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects: TETRAETHYL LEAD - Nerve toxin, small amounts are fatal For much more specific information about Gasoline sources, Gasoline toxicology, Gasoline MSDS, Gasoline FAQs, and public health information please visit our Extensive Guide to Gasoline.
GLASS & WINDOW CLEANERSWindow and glass cleaner commonly contains isopropyl alcohol or Ammonia, water, and coloring. It may be mildly irritating to the eyes, skin, nose, and throat. Use: Always use window and glass cleaners in a well-ventilated area. Storage: Keep out of reach of children. Disposal: Unused or unwanted portions of window or glass cleaner should be flushed down the drain with plenty of water. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
AMMONIA - Fumes irritate eyes, lungs; can cause burns or rashes on skin
GLUES & ADHESIVESGlues, rubber cement, epoxy, and other adhesives contain a solvent which, when applied, evaporates out leaving the solid adhesive portion behind. Rubber cement, epoxy, instant glues, model glues, and plastic adhesives contains five dangerous solvents. Many adhesives are extremely flammable. Some adhesives are skin and lung irritants and allergy-sensitizers while others can cause burns to skin and eyes. Many of the solvents used in adhesives and glues have narcotic, possibly fatal, effects when inhaled in high concentrations. Inhalation of fumes from cured epoxy resins may result in coughing and bronchial spasms for several days. Instant glues contain small amounts of solvent which rapidly dissolve. This product is considered to be of low toxicity, but there are other concerns to be noted. Instant glues bond so rapidly and strongly that the least sloppiness can result in an undesirable and sometimes dangerous situation. A drop of glue on the finger followed by a touch to the eye can end with a semi-permanent finger in the eye (which will release in less than 24 hours). The safest glues on the market are white glue, library paste, yellow wood glue, and glue sticks. White glue effectively bonds most porous and semi-porous materials such as paper, cloth, wood, and pottery. White glue can also be used for big jobs such as laying hardwood floors. Use: Use white glue, glue sticks, or yellow glue when ever possible. Never use toxic adhesives on laminated cutting boards, bowls, or a product which contacts food. Carefully read the label. Wear protective gloves with adhesives and cements. If the glue contains solvents, use only in a well ventilated area with plenty of fresh air. Avoid wearing soft contacts, which may absorb solvent vapors. If the adhesive is flammable be certain to extinguish sources of ignition (such as pilot lights) if you will be using a large quantity of the solvent in a room where a source of flame is located. Keep the lid tightly closed when the glue is not in use. Storage: Store away from children and sources of flame. Make sure cap or lid is tightly secured. Disposal: The best way to avoid a waste disposal problem is to use it up as intended. If the adhesive or glue is anything other than instant, white, or yellow glue, it is recommended that this product be disposed of by a licensed hazardous waste handler or saved for a household hazardous waste collection. However, if the glue or adhesive has hardened, it may be thrown in the trash destined for the landfill. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
NAPHTHALENE - Damages liver, prolonged vapor exposure has led to cataract formation
HAIR COLORHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
CADMIUM CHLORIDE - Fumes irritate eyes, lungs; can cause burns or rashes on skin
HAIR PERMANENTHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
AMMONIUM THIOGLYCOLATE - Causes skin rash and hemorrhages under skin; hypoglycemia has been associated with toxic exposure
HAIR SPRAYHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
AEROSOLS - Either associated with brain damage or highly flammable
HYDROFLUORIC ACIDHydrofluoric acid is a highly toxic, highly corrosive, colorless, fuming liquid found in many aluminum cleaners. It's one of the few acids that is fatal with only a small area of skin exposure. It's such a powerful acid that it will even etch highly stable glass, alumina, and titanium materials. Hydrofluoric acid acts differently from all other acids. The onset of injury proceeds inconspicuously but with grave effects. Hydrofluoric acid is highly corrosive to the skin and can produce first-degree burns. The pain from burns may be delayed for several hours, during which time the acid will burn deep into the skin. Burns may not become apparent for one to twenty-four hours and may appear as reddened, pasty white, blistered, or charred spots. Hydrofluoric acid can damage muscles, ligaments, and bone in its progression after skin exposure. I can attest to the extreme dangers of Hydrofluoric Acid because we use it on a daily basis in our Inorganic labs. Hydrofluoric Acid and Fluoboric or Fluoroboric Acid are some of the few acids which will dissolve the silica and alumina based chemical catalysts we analyze. We take special precuations when using Hydrofluoric Acid because unlike the other acids we use, if enough Hydrofluoric Acid contacts the skin it can be deadly! Many have died from relatively small skin surface area exposure to Hydrofluoric Acid. It's an extremely painful way to die! Throughout our labs we have easily accessible vials of Calcium Gluconate which is a cream to be immediately applied upon any skin contact with Hydrofluoric Acid. Calcium Gluconate quenches the reaction of Hydrofluoric Acid with the body's calcium. It's an essential item to have around when using Hydrofluoric Acid because if exposure to Hydrofluoric Acid occurs a pernicious chain reaction ensues which affects tissue and blood eventually resulting in severe damage and likely death. Because our labs use this deadly acid on a daily basis we have notified the authorities so they can be prepared in the event of an emergency due to Hydrofluoric Acid exposure. The local emergency response teams, our own first responder emergency management teams, and the local hospitals have all been equipped with special injectable antidotes and the Calcium Gluconate cream which counteract the effects of Hydrofluoric Acid exposure. STAY AWAY FROM HYDROFLUORIC ACID! IT'S A KILLER! Use: Do not use products with hydrofluoric acid. If the aluminum cleaner ingredients are not on the label, you cannot assume hydrofluoric acid is not in the product. If you are using a product which contains this ingredient, protect all exposed skin in addition to wearing protective gloves, safety goggles, and a respirator with an acid gas cartridge.
HYDROGEN PEROXIDEHydrogen peroxide is a clear, colorless liquid. Common household hydrogen peroxide contains a 3-5% concentration. It is used as a disinfectant and deodorizer. However, the benefit is of short duration. In general, ingestion or skin exposure of small amounts of household hydrogen peroxide will cause no serious problems. It is mildly irritating to the skin and mucous membranes and causes a whitish discoloration. Industrial strength hydrogen peroxide used as a wood or hair bleaching agents (10% concentration H2O2) may result in severe burns to the skin, throat, and gastrointestinal tract. Disposal: Unused or unwanted portions of household hydrogen peroxide should taken to a hazardous household waste collection center. If a collection center is not available, 3-5 % peroxide solutions can be flushed down the drain. If you use a septic tank or lagoon, dispose of small quantities over several days. For information about disposing of 10% peroxide solutions, contact your local fire department or wastewater treatment plant.
INSECT BATES FOR ANTS, COCKROACHES, AND CRICKETS
What is it? For insect baits to work, areas where food is stored, prepared or eaten need to be kept clean. Because, if there are other foods around that the insect likes better, or finds first, it will probably not eat the bait at all. So do baits kill just one insect at a time? No. Baits work by "tricking" the insect into eating something poisonous and spreading the poison to others. How do they spread the poison? Both ants and cockroaches leave a scent trail for others to follow to find the bait. Also, ants may carry some of the bait back to their colony to share with other ants. In a short time the insecticide kills the insects who have eaten the bait. But how fast a bait works depends on several things. It depends on the kind of pesticide in the bait, whether the insect likes the taste of the bait and whether there is other food around for the insect to eat instead. You may have seen insect bait containers on counter tops, in cabinets, hidden behind stoves or refrigerators or on the floor near cracks or crevices where insects go in and out. They are usually square or round with a flat top, and about half an inch high. They may also be sort of dome shaped like an igloo. The containers are about two (2) inches across in size and may be plastic or metal. The bait inside the container is usually a solid or a gel. Some baits aren't in a container at all. They can be tablets or gels that are put out for insects like cockroaches to eat. Always "Read the Label First" to know how to properly use these products and for safety information.
What's in it?
What health and safety things do you need to think about with insect baits?
INSECTICIDESHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
ORGANOPHOSPHATES - carcinogenic in rats; teratogenic in chick embryos; affects nervous system; acutely toxic causing headache, dizziness; twitching, nausea
INSECT REPELLENTSAs the name indicates, insect repellents deter mosquitoes, gnats, and other insects from biting and annoying the user. Common active ingredients in repellents include: Diethyl toluanide, Dimethyl phthlate, Ethyl hexanediol, Indalone, Di-n-propylisocinchoronate, Bicycloheptene dicarboximide, and Tetrahydro furaldehyde. The literature reports at least five cases of toxic exposures due to excessive skin absorption of diethyltoluamide (DEET), a common ingredient in twelve of the fifteen insect repellents examined by Consumers Union. Symptoms in all cases included loss of coordination, anxiety, behavioral changes, and mental confusion. Liver and kidney damage have been linked to indalone and ethyl hexanediol. Long-term skin application of indalone has caused liver and kidney damage in animals. Ethyl hexanediol may cause liver and kidney damage. Ingestion of large doses of insect repellent may cause loss of coordination, central nervous system depression, and possibly coma. Use: Use sparingly. Avoid contact with eyes, mouth, and sensitive skin. Storage: Keep out of the reach of children. Disposal: Insect repellents are pesticides. With the exception of insect repellents containing banned or otherwise restricted pesticides, the best way to get rid of them is to use them up as intended. Insect repellents should never be burned, buried, mixed together, poured on the ground, dumped in the water, or poured down the drain. Leftover portions of all pesticides, including insect repellents, must be disposed of by a licensed hazardous waste handler or through a professional household hazardous waste collection. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
BUTOPYRONOXYL - Can cause mild necrosis in liver and kidney
INSECT SPRAY
What is it? Insecticides used around your home usually come in the form of liquids, sprays or powders. Sometimes they are mixed with other products that are used around your house. Sometimes they are mixed with other pesticides. For example a fertilizer for your grass may have an insecticide in it. It could even have both an insecticide and a herbicide (weed killer) in it.
What's in it?
What health and safety things do you need to think about with insecticides? Insecticides can hurt your eyes. They can make you really sick if you breathe their fumes, get some in your mouth or on your skin and you don't wash it off right away. They can also be fatal. How you are effected depends on the route of exposure.
ISOPROPYL ALCOHOLIsopropyl alcohol, also known as isopropanol, is a colorless liquid with a pleasant odor. It is highly flammable. Isopropyl alcohol is found in alcohol sponges, cleaning agents, and rubbing alcohol (though some rubbing alcohols contain ethanol), and is a good disinfectant. Most rubbing alcohol contains 70% isopropyl alcohol. Poisoning can occur through skin absorption, oral ingestion, or inhalation. Symptoms from ingestion, inhalation or absorption of large quantities include flushing, headache, dizziness, mental depression, nausea, vomiting, anesthesia, and coma. Alcohol baths or sponges to soothe a fever can lead to acute poisoning through skin absorption or inhalation. Instead, the Regional Poison Center suggests using tepid water as a sponge bath to fight fever. Use: Wear protective gloves when using (see "Household Safety Equipment"). Use in well-ventilated areas. Storage: Keep out of the reach of children and pets. Make sure lid is tightly capped. Store away from sources of flame or ignition. Disposal: Flush down drain with plenty of water. If you have a sewage tank or lagoon, dispose of small quantities over a number of days.
LATEX PAINT
What is it?
What's in it?
What health and safety precautions do you need to think about with latex paint? Before using these products, you need to be sure to always read the label first to know how to properly use these products and for safety information. In the room that is being painted, open the windows and doors fully. Put a box fan in the window directing the air and fumes out of doors. Keep the fan on while painting and for about 48 hours thereafter. Keep small children away from the room where the painting is being done and away from the open cans of paint. Do not use paint that is labeled for "exterior use only" indoors.
LEADSince 1993 lead has been effectively banned from Gasoline sold in the United State, leading to a drastic decrease in airborne lead pollution. Elsewhere, it is estimated that 70 to 90% of the lead burned in goes out the tailpipe and ends up as particulate matter in the air. Lead in the air is very dangerous because it is directly absorbed by the body as an inhaled material. The primary sources of lead poisoning are from the ingestion of lead particulates in the air and the ingestion of lead-based paints which may be found in older homes, in paints on toys and playground furniture, and in decals on drinking glasses. Lead can also be found in pottery and ceramics, dust in topsoil, factory and automobile fumes, fumes from battery casings, water from lead pipes, and in lead weights. One small paint chip, half an inch square, can contain more than 1 milligram of lead - 10 times more than the maximum "safe" daily dose of lead. Since 1977, household paint cannot contain more than 0.06% lead by dry weight. However, high lead contents are still allowable in automobile paint, bridge paint, heavy equipment paint, street markings, and any non-residential paint. The lead limit in these paints can be whatever amount is deemed necessary by the manufacturer. Lead is poisonous in all forms. It is one of the most hazardous, toxic metals because once in the body it can accumulate. Ingestion and inhalation of lead cause the most severe symptoms. Its symptoms are many and severe. Among the symptoms of lead poisoning are leg cramps, muscle weakness, irritability, lethargy, stupor, behavioral disturbances, hyperexcitability, convulsions, brain damage, anemia, weight loss, and malnutrition. Other symptoms include headaches, sterility, miscarriage, or the birth of brain-damaged babies. To avoid accidental lead poisoning in your home, follow these suggestions:
For questions pertaining to lead or to test your paint for lead content, contact the nearest State Department of Health Office. For questions concerning lead in your tap water, contact your city or county water department. For much more specific information about sources of lead, FAQs about Lead, hazards, toxicology, MSDS, and public health Q and A, visit our Extensive Guide to Lead.
LYELye, also known as caustic soda, sodium hydroxide, potassium hydroxide, and caustic potash, is commonly used in drain cleaner, oven cleaner, and in some non-phosphate detergents. Lye is extremely caustic. Its chemical action eats away materials (including skin tissue). Contact with skin or mucous membranes causes burns and frequently deep ulcerations with scarring. Mists, vapors, and dust can cause small burns. Eye contact causes severe damage, including blindness. Use: Caustic products containing lye should be properly labeled with the words "Danger" and "Poison" to indicate their dangerous nature. Lye-based liquids usually contain a warning to avoid squeezing the container, but carelessness could lead to a disfiguring splash on the skin or a blinding squirt in the eye. Products that contain lye in a pellet form sometimes require you to measure a spoonful out of an opening which is too small for a spoon to fit. This situation is very hazardous because lye-based pellets are easily spilled as one pours the product from the container onto the spoon. Be extremely careful when using lye-based products. Wear gloves and goggles in addition to protecting exposed skin. Avoid fumes by using only under conditions where adequate ventilation exists. Immediately wipe up spilled lye and wash off with plenty of water. Storage: Keep products containing lye away from children and pets.
MERCURYElemental mercury, the form found in thermometers, thermostats and other household products, creates hazardous vapors at room temperature. Exposure to very high mercury vapors can cause a variety of symptoms including chronic inflammation of mouth and gums, personality change, nervousness, fever, or a rash. To avoid accidental mercury poisoning in your home, follow these tips:
For much more specific information about mercury sources, mercury in fish, mercury toxicology, mercury MSDS, mercury FAQs, and public health please visit our Extensive Guide to Mercury
METHANOLMethanol is made from the distillation of wood. Synonyms are methyl alcohol, wood alcohol, wood spirits, or curbinol. Methanol is used primarily in antifreeze compounds, paints, cements, inks, varnishes, shellacs, wood strippers, windshield wiper solvents, gasoline antifreeze and as a solvent in dyes. It is also used as a fuel in Sterno (4% methanol), home heating oil extenders, and may be added to Gasoline in place of ethanol to make gasohol.Methanol is highly toxic and readily absorbed from a routes of exposure. Symptoms include malaise, headache, dizziness, confusion, abdominal cramps with excruciating pain and tenderness, stupor, weakness, and acidosis. When methanol is swallowed, Formaldehyde is metabolically generated. This formaldehyde is more toxic than the methanol itself. Blindness and death may occur following ingestion. When using any product which contains methanol, be certain to wear gloves. Provide adequate ventilation, making sure that vapors are blowing away from you. To dispose of products that contain methanol, save for a household hazardous waste collection rather than flushing them down the drain or throwing them in the trash.
METHYLENE CHLORIDEMethylene chloride, known also as methylene dichloride and dichloromethane, is a colorless, volatile liquid with an ether-like odor. It is commonly found in septic tank cleaners, paint and varnish removers, degreasers, pesticides, aerosols, and Christmas bubble lights.Methylene chloride irritates skin that comes in contact. When inhaled, it mimics carbon monoxide toxicity. Memory loss and liver and kidney damage are reported with chronic exposure. Methylene chloride is a known animal carcinogen and a suspected human carcinogen. When heated, methylene chloride emits a highly toxic phosgene gas (nerve gas). The use of products containing methylene chloride by people with heart conditions has resulted in fatal heart attacks.
MILDEW REMOVERHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
PHENOL - Central nervous system depression; severely affects circulatory system; corrosive to skin; suspected carcinogen Borax is an effective mildew remover and short-term preventive.
MOTHBALLSMothballs are a distinctive smelling, volatile solid used to repel moths. Mothballs, which are classified as a pesticide, may look like candy to a child. They are poisonous when eaten and seizures can develop in less than one hour. Mothballs contain 100% of either naphthalene or paradichlorobenzene. Both of these ingredients can produce harmful effects when they enter your system through inhalation. Irritation to nose, throat, and lungs, headache, confusion, excitement or depression, and liver and kidney damage can result from exposure to mothball vapors over a long period of time. Mothballs containing naphthalene are of special concern because naphthalene can promote a breakdown of red blood cells resulting in hemolytic anemia. Hemolytic anemia in mild form may cause only fatigue. In more severe cases, it can cause acute kidney failure. Young children are at particular risk. Poisonings have been reported following dressing infants in clothing that was stored with naphthalene mothballs, suggesting that absorption of naphthalene may occur through the skin. The warning label on mothball products reads "avoid prolonged breathing of vapors." This label is at odds with the normal use of mothballs. By the very nature of their ingredients, mothballs give off strong odors (vapors which you can smell). These vapors tend to fill the entire home, making it nearly impossible to avoid prolonged breathing of vapors unless you live outdoors. The situation is complicated further when mothballs are placed in closets or rooms with poor ventilation, where the vapors build to high concentrations. Vapors are absorbed by clothes, blankets, and sheets resulting in direct exposure when you are around these items. Use: Avoid these products. If you do use mothballs, use them sparingly. Mothballs which contain paradichlorobenzene may be safer, if only because they do not promote hemolytic anemia. Storage: Store away from children and pets in a well ventilated area. Mothballs, if stored indoors, should be tightly wrapped in two plastic bags. Disposal: Mothballs should be taken to a licensed hazardous waste handler or saved for a professional household hazardous waste collection program. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
PARADICHLOROBENZENE - Vapor irritates skin, eyes and respiratory tract; large doses can cause injury to liver; suspected carcinogen
MOTOR OILMotor oil is a petroleum distillate product composed of 75% mineral oil, 20% oxidation inhibitors and detergents, and 5% pour depressants and viscosity improvers. Used or waste motor oil is often contaminated with Lead outside the United States (from Gasoline), magnesium, copper, zinc, and other heavy metals which are picked up from the engine. Used motor oil can present a threat to health through skin contact, skin absorption, inhalation, or ingestion. Many of the problems associated with used motor oil are due to exposure to the heavy metals. These health problems are cumulative, so with each exposure to used motor oil the amount of heavy metals added to the body's system increases. Used motor oil poses a very serious threat to the environment when disposed of improperly. Just one gallon of motor oil can pollute one million gallons of water and can form an oil slick nearly 8 acres in size! Use: Wear protective gloves. Storage: Store away from children and sources of ignition. Place used motor oil in a labeled container with a tight-fitting lid (a plastic milk jug with a tight-fitting cap works well). Disposal: Please follow these directions to avoid harming our environment and groundwater:
HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
HEAVY METALS - Cause nerve and kidney damage, suspected carcinogen
NAIL POLISHHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
Toluene - Produces headache, nausea, narcosis, central nervous system depression
NAIL POLISH REMOVERHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
Acetone - Toxic if ingested; irritates lungs, causes nails to become brittle; flammable
NAPHTHASNaphthas, which are flammable, irritant, and toxic, are derived from both petroleum distillation and coal tar. Naphthas are used as organic solvents for dissolving or softening waxes, oils, greases, varnishes, and plastics. The less flammable fractions are used in dry cleaning. Heavy naphtha serves as a base for insecticides. Mineral spirits are also included under naphthas. Naphthas can enter into your system through inhalation and vapors, ingestion, and eye and skin contact. Naphthas are irritating to the skin, eyes, and upper respiratory tract. Skin chapping and sensitivity to light may develop after repeated contact. Petroleum naphtha has a lower order of toxicity than coal tar naphtha. Overexposure to either type of naphtha may cause central nervous system depression with symptoms of inebriation followed by headache and nausea. When using a product that contains naphtha, be certain to wear gloves. Provide for adequate ventilation, making sure that the fumes are blowing away from you.
NAPHTHALENENaphthalene, also known as tar camphor, is a white crystalline solid with a distinctive mothball odor. Naphthalene is available to the public as a pest repellent and is frequently contained in mothballs, mothflakes, and toilet bowl deodorizers. Napthalene can enter your system through inhalation, skin absorption, ingestion, and eye and skin contact. Napthalene may produce possible damage to eyes, liver, kidneys, skin, red blood cells, and the central nervous system. Hemolytic anemia, caused by the breakdown of the red blood cells, has been reported following immediate and long-term exposure. Infants exposed to clothes, blankets, and diapers stored in naphthalene mothballs are at risk for hemolytic anemia. Mild degrees of anemia often cause only slight symptoms like a lack of energy and fatigue. In more severe cases, hemolytic anemia can cause acute kidney failure.
NITROBENZENENitrobenzene, a highly toxic substance, is a pale yellow to dark brown oily liquid whose odor resembles bitter almonds or black paste shoe polish. Nitrobenzene is found in some shoe polish, furniture polish and floor polish, leather dressings, paint solvents, gun bluing, and is sometimes used to mask unpleasant odors. Nitrobenzene can enter your body through inhalation, skin and eye contact, and ingestion. It affects the central nervous system, producing fatigue, headache, vertigo, general weakness, and in some cases severe depression, unconsciousness, and coma. Drinking alcohol increases the toxic effects of nitrobenzene. Chronic exposure to nitrobenzene may lead to spleen and liver damage. At increased risk are pregnant women (due to the ability of nitrobenzene to cross the placenta), individuals consuming alcoholic beverages, and individuals with glucose-6-phosphate-dehydrogenase enzyme deficiency. Whenever possible, avoid products that contain nitrobenzene.
OIL-BASED PAINTS
What is it?
What's in it?
What health and safety precautions do you need to think about with oil based paint? Before using these products, you need to be sure to always read the label first to know how to properly use these products and for safety information. In the room that is being painted, open the windows and doors fully. Put a box fan in the window directing the air and fumes out of doors. Keep the fan on while painting and for about 48 hours thereafter. Keep small children away from the room where the painting is being done and away from the open cans of paint. Do not use paint that is labeled "for exterior use only" indoors. If the room you are painting does not have a window, consider using a latex paint.
OVEN CLEANERThe majority of oven cleaners contain lye (sodium hydroxide or potassium hydroxide), which is an extremely corrosive ingredient. Whether the cleaners are contained in aerosol spray form, liquid, paste or powder, lye can attack skin, eyes, or internal organs. Lye in aerosol form is especially hazardous because small droplets containing lye can drift and land on skin, eyes, and sensitive lung surfaces. Labels on most oven cleaners warn that the product can burn skin and eyes and that fumes and vapors should be avoided. Use: Avoid aerosol oven cleaners. Wear an apron, protective gloves, safety goggles, and a respirator with an organic vapor cartridge. Make sure there is plenty of fresh air and adequate ventilation present. Storage: Keep out of the reach of children. Disposal: Use up as intended. Take unused portions to a hazardous household waste collection center. If a collection center is unavailable, wrap in several layers of newspaper and dispose of in the trash. Alternatives: Use a non-toxic oven cleaner. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
SODIUM HYDROXIDE - Extremely corrosive, burns skin and eyes; usually fatal if swallowed; aerosols disperse chemicals, increasing inhalation dangers
PAINTThe typical paint mixture is 5-25% pigment and 75-95% solvent. The type of pigment and solvent used largely defines the toxicity of the paint. Paints may become hazardous if fumes are inhaled or if paint is ingested. Another hazard associated with some paints is flammability. The label should state whether or not the paint you are using is flammable. With the exception of latex paint, which has water as a solvent, solvents commonly used in paints include mineral spirits (naphtha), Toluene, Xylene, and other petroleum distillate solvents. These solvents can irritate your eyes, skin, and lungs. Inhaling paint fumes can result in headaches, nausea, dizziness, and fatigue. Toxic fumes can accumulate in closed spaces and areas with poor ventilation. Acute and chronic symptoms include muscle weakness, liver and kidney damage, and respiratory problems. Due to the high solvent content of oil-based paints and varnishes, women should avoid using these products while pregnant. Indoor water-soluble latex paints may be of low toxicity unless ingested in large quantities. Exterior latex paint may contain a Mercury pesticide to provide mildew resistance which could be highly toxic if ingested. Use: If possible, use latex paint rather than oil-based or other paints that require a solvent to clean up. Not only will you eliminate the hazards from the solvents in the paint, you will eliminate the need to use additional solvents to clean brushes. Wear protective gloves. If you need to clean oil-base paint from your skin, massage with a few drops of baby oil, butter, or margarine. Wipe dry and wash with soap and water. There are now many no VOC, low VOC, and non-toxic paint brands available which create a significantly reduced air quality, use, and disposal hazard. These low toxicity paints often lack the dangerous Volatile Organic Compounds such as Toluene and Xylene. Therefore, volatile solvent fumes which made traditional paint formulations so toxic have been reduced or eliminated. Vinyl compounds may also be greatly lower, thus reducing that "new home smell" after painting with these non-toxic paints. That's a good thing since that "new home smell" like "new car smells" mean you are breathing toxic and often carcinogenic chemicals! Whenever possible, paint outdoors. When painting inside make sure ventilation is adequate. Use a fan to direct fumes away from the area where you are working and to the out-of-doors. Take plenty of fresh air breaks. Do not place flammable paints near flames, sources of sparks, or areas of intense heat. Never smoke around paints or while painting. Your paint is usable if it will mix up when stirred. Oil paint can be usable for up to 15 years. Latex paint is usable if it is less than 10 years old and has not been repeatedly frozen and thawed. To see if your latex paint is still usable after being frozen, brush it onto newspaper and see if there are lumps. Paint is not usable if lumpy. Storage: Seal can tightly when not in use. Keep all paints and paint products away from children and pets. Store flammable paint away from heat, flame, and source of ignition. Do not allow paint to freeze. Disposal: The best way to dispose of paint is to use it up. Some suggestions to use up old paint are to paint boards, signs, dog and bird houses, or use it as an under coat for another project. Paint can be recycled if there is more than one-third gallon of usable paint and the paint is in the original can with a legible label. If you have useable recyclable paint and you cannot use it, recycle it by giving the paint to someone who can use it, such as friends, neighbors, schools, and theater groups. If your paint has completely dried inside the paint can, can be placed in the trash destined for the sanitary landfill. If you have liquid paint which cannot be used or recycled, secure and hold for a professional household hazardous waste collection or give to a licensed hazardous waste handler. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
ORGANIC SOLVENT - Irritating to eyes and skin, can cause cracking of skin and depression of nervous system
PAINT THINNERTurpentine and mineral spirits are commonly used in thinning paints and varnishes. Both ingredients are flammable and toxic, though mineral spirits are of lower toxicity. Mineral spirits, a petroleum distillate, can be harmful through inhalation, skin and eye contact, and ingestion. Contact and inhalation can cause eye, nose, and throat irritation, dizziness, and dermatitis. Ingestion can induce central nervous system depression. Damage to lungs may result if mineral spirits are swallowed and then vomited. Turpentine, a sticky mixture of resin and oil obtained from pine trees, is an irritating substance that can cause tissue death as well as damage to kidneys. Intoxication from vapors produces central nervous system depression with possible symptoms of headache, nausea, confusion and disturbed vision. Continued inhalation of vapors can cause a predisposition to pneumonia and chronic kidney inflammation. Vapors even in low concentrations can irritate eyes, nose, and throat. Use: Wear a respirator with an organic vapor cartridge and protective gloves. Use paint thinners only if ventilation is adequate and take plenty of fresh air breaks. If skin contact occurs, wash the area immediately with soap and water. Storage: Keep out of the reach of children. Store in a well-ventilated area away from flames and sources of ignition. Disposal: Dirty paint thinner can easily be recycled at home for reuse. Pour the dirty paint thinner into a clearly labeled container with a good seal. Plastic jugs such as milk jugs may not be strong enough to withstand the vapor pressure in a warm environment. Glass jars work well but never use a beverage container because it can be easily mistaken for something to drink. Clearly label the container with the type of solvent and the date. Draw or write a clearly visible warning (such as a skull and crossbones or the word Danger). Store it away from sources of sparks for several weeks to months until the paint sludge settles on the bottom. Carefully pour the clean solvent off the top. This solvent can be reused. Allow the remaining paint sludge to dry completely in a well-ventilated area, outside of your home and away from pets and children. When all of the liquids have evaporated, the hardened sludge can be discarded in the trash. Small amounts of dirty paint solvent can be poured into a paint can of the same color and mixed well. This thinned paint can then be used for a second coat or another project. The best way to get rid of left over paint thinners is to use them as intended or find someone else who will. Unwanted portions of mineral licensed hazardous waste handler or a professional household hazardous waste collection. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
Toluene - Produces headaches, nausea, narcosis, central nervous system depression
PAINT & VARNISH REMOVERThere are a variety of different formulations for products that remove paint and varnish (also called paint and varnish strippers). Most paint and varnish removers contain organic solvents which are hazardous to human health. Most are highly flammable. Some nonflammable products will produce a toxic gas when in contact with flame. Paint and varnish removers may contain some of these hazardous ingredients: Acetone, Benzene, isopropyl alcohol, methanol, methylene chloride, petroleum distillates, Toluene, trichloroethane, and Xylene. Although not presently used in paint and varnish removers, Benzene, a known human carcinogen, was an ingredient in older products. Hazardous ingredients in paint and varnish removers can harm your body through skin contact, skin absorption, ingestion, and inhalation. A common ingredient, methylene chloride, is a powerful narcotic which break down in the body to form carbon monoxide, potentially resulting in oxygen deprivation. The use of paint and varnish removers containing methylene chloride by people with heart conditions has resulted in fatal heart attacks. Methylene chloride is also a known animal carcinogen and a suspected human carcinogen. See the specific ingredients in this section for additional information on the hazards associated with paint and varnish removers. Use: Never use paint and varnish removers containing Benzene. If you have a heart condition, do not use products containing methylene chloride. Follow label directions carefully. Do not smoke while using these products. Do not use paint and varnish removers near flames, sparks, sources of ignition, or areas of intense heat. Beware of using paint and varnish removers when the gas furnace is operating. The vapors may destroy your furnace by corrosion and the pilot light can ignite the vapors which will then explode. Wear protective gloves and safety goggles. Work outdoors and in the shade. If you must work indoors, be sure to have adequate ventilation. Take plenty of fresh air breaks. If you can smell the product, you are inhaling the solvents and should wear an approved respirator with an organic solvent cartridge. Never use paint and varnish remover to clean your hands. (To remove oil-based paint from skin, massage with a few drops of baby oil, butter, or margarine. Wipe dry and wash with soap and water.) When through working, wash your hands and all exposed skin thoroughly before eating and drinking. When you are finished for the day, place solvent-covered rags and newspapers into a metal container with a lid and place the container outside of the house. Place the container beside your household trash for pick-up. Storage: Store out of reach of children and pets in a cool, dry, well-ventilated area. Keep containers tightly closed. Keep away from sources of sparks, ignition, and flame. Disposal: The best way to dispose of leftover paint and varnish remover is to use it up as intended or find someone who will use it up. If you cannot find a donation outlet for the paint and varnish remover or if it contains Benzene, store and hold the product for a professional household hazardous waste collection or give it to a licensed hazardous waste handler for disposal. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
Benzene - Destroys ability to produce blood cells, can cause leukemia; flammable; carcinogen
PARADICHLOROBENZENEParadichlorobenzene is a white solid crystal with a wet oily surface. It is volatile and gives off penetrating mothball-like odors. Paradichlorobenzene is commonly found in mothballs, moth crystals, and in diaper, toilet, and room deodorizers. Inhalation may result in headache, swollen eyes, stuffy head, anorexia (loss of appetite), nausea, vomiting, and throat and eye irritation. With prolonged skin contact, allergies and skin irritation have been reported. Symptoms from ingestion include nausea, vomiting, diarrhea, liver and kidney damage, and methemoglobianemia (which interferes with the uptake of oxygen). The lack of convincing reports of human and animal toxicity supports the notion that paradichlorobenzene has a lower order of acute toxicity than naphthalene, which is also commonly used as a moth repellent.
PERCHLOROETHYLENEPerchloroethylene, also known as tetrachloroethylene, ethylene tetrachloride, or PERC, is a chlorinated hydrocarbon solvent commonly used in dry-cleaning fluid, spot removers, and degreasers. Vapors are irritating to skin, eyes, and upper respiratory tract. Inhalation exposure produces giddiness, headache, inebriation, nausea, vomiting, and sinus inflammation. Skin exposure will cause redness and chapping. If ingested, perchloroethylene can result in central nervous system depression and liver damage. Chronic exposure may also result in liver damage. Perchloroethylene is a chlorinated hydrocarbon solvent that slowly breaks down in the environment. It is fat soluble which allows it to collect in the tissues of living organisms and accumulate in the environment. Perchloroethylene is a known animal carcinogen that has caused liver cancer in mice.
PET SUPPLIES(Flea Collar, Shampoo, Spray, Powder) HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
ORGANOPHOSPHATES - Carcinogenic in rats, teratogenic in chick embryos; affects nervous system; acutely toxic causing headache, dizziness, twitching, nausea
PETROLEUM DISTILLATESPetroleum, a thick natural oil obtained from beneath the earth, consists of various hydrocarbons, a class of chemicals containing hydrogens and carbons. Petroleum distillates, also called hydrocarbons or petrochemicals, refer to a broad range of compounds which are extracted by distillation during the refining of crude oil. During the fractional distillation of petroleum, crude oil is heated to allow various compounds to turn from liquid into gas and then captured as they rise, cool, and condense. Lighter, more volatile compounds rise higher before they condense and are collected on distillation trays. Heavier, less volatile compounds such as diesel fuel and oil are collected on lower distillation trays. Waxes and asphalts are collected from the bottom after the other products have volatilized. Petroleum distillates are found in a wide variety of consumer-products including lip gloss, liquid gas, fertilizer, furniture polish, pesticides, plastics, paint thinners, solvents, motor oil, fuels and hundreds of other products. Petroleum distillates listed commonly on labels of general household products are those that distill off around naphthas. Petroleum jelly, a petroleum distillate product, is generally regarded as non-toxic. Petroleum distillates contain both aromatic hydrocarbons (carbon rings) and aliphatic hydrocarbons (straight carbon chains). The chemical structure of the hydrocarbon largely defines the nature and behavior of these compounds. Aromatic hydrocarbons are the most toxic compounds found in petroleum products. Most aromatic hydrocarbons are long-term toxins and known cancer causing agents. These aromatic compounds are found in all crude oils and most petroleum products. Many aromatic hydrocarbons have a pleasant odor and include such substances as naphthalene, Xylene, Toluene, and Benzene. Aliphatic hydrocarbons are flammable and may be explosively flammable. Aliphatic hydrocarbons include methane, propane, and kerosene. Aliphatics and aromatics pose a special health risk if ingested and vomited. When swallowed, the lighter, more volatile distillate products can be sucked into the lungs interfering with the lung's functions and chemical pneumonia may result. Aspiration of fluid into the lungs can occur both during swallowing and vomiting of the product. Upon skin contact, petroleum distillates can produce local skin irritation and sensitivity to light in some individuals. Environmentally, many of the petroleum distillate products add to smog and water pollution due to improper disposal or during their manufacture and use. Products which contain petroleum distillates should be used carefully. Wear gloves to avoid skin contact and avoid breathing vapors of volatile compounds. Always keep petroleum distillate products out of reach of children. Do not mix different petroleum distillate products. Refer to the specific petroleum distillate product listed in this guide for safe use, storage, and disposal information.
PHARMACEUTICALSPharmaceuticals, which include both prescription medicines and over-the-counter drugs, can be disposed of easily and safely. The best way to dispose of pharmaceuticals is to return the unwanted portion to your pharmacist. Many pharmacists are willing to accept and properly dispose of unwanted pharmaceuticals. The second best alternative is take the drugs to a hazardous household collection center. If a collection center is unavailable, many medicines can be flushed down a toilet connected to a sanitary sewer. NOTE: this excludes chemotherapy drugs, antineoplastic medicines, and shampoos for head lice, which should never be disposed of down a toilet. If you have a septic tank or lagoon, return your unwanted prescriptions to the pharmacist or take to a household connected to the sanitary sewer. Do not place pills in the trash where children could possibly try them with dire consequences. Unwanted chemotherapy drugs and antineoplastic medicines should be returned to the pharmacist or the cancer clinic where the drugs were administered or taken to a major hospital for proper disposal. These drugs are extremely toxic. Some shampoos for head lice contain the insecticide lindane. Therefore, unwanted quantities of these shampoos should be held and stored for hazardous waste collection. Disposal: Disposable hypodermic syringes and needles are used in many homes to administer medications such as allergy shots and insulin. Improper disposal of the needles can injure waste handlers. Dispose of the hypodermic needles by placing them in a rigid, puncture resistant, leak-proof container. When the container is full, seal it with duct tape and place the container in the trash destined for the sanitary landfill.
PHENOLPhenol, also known as carbolic acid, is flammable, corrosive, and very toxic. Phenolic compounds have a distinct odor and are used in disinfectants, deodorizers, paints, and as anesthetic for skin. Ingestion of even small amounts may cause vomiting, circulatory collapse, paralysis, convulsions, and coma. Light sensitivity and sinus congestion are common with exposure to fluids or vapors. Fatal poising can occur through skin absorption. Phenol and related compounds rapidly denature all proteins they come in contact with, including skin. Severe burns may occur upon contact. A concentration of 1% phenol, used to prevent itching from insect bites and sunburn, applied over several hours, was reported to cause gangrene in one individual. Skin ulcerations, skin rashes, swelling, pimples, and hives have been widely reported. The anesthetic properties of phenols can allow extensive damage to skin tissue before pain is perceived. Although there have been many poisonings from phenolic solutions, phenol continues to be used in consumer products.
PHOTOGRAPHY CHEMICALSPhotography chemicals are substances used for processing film and making prints. This category includes a wide variety of chemicals. The largest manufacturer of photography chemicals, Kodak, has more than 20,000 products currently on the market. The photography chemicals most often used at home are those involved in black-and-white film processing. The most commonly used solutions are developer, fixer, and stop bath. Photography chemicals that require special handling include intensifiers, dyes, and toners, which may contain selenium, uranium, iron, gold, and platinum. Color film processing is more complex. In particular, the developing baths of color transparency and color negative processing and home color printing require special precautions. Many chemicals used to develop photographs are corrosive and can cause skin, eye, and lung irritation. Inhalation and skin contact are the primary routes of hazardous exposure. These chemicals are toxic if swallowed. Acids used in developing can burn and blind you. Products which contain Benzene, a known cancer causing agent in humans, can be especially hazardous Photography chemicals have a longer shelf life in a powder form than in liquid concentrate, but the powder Powdered chemicals require longer agitation completely dissolve, possibly forming vapor droplets. These droplets are easily inhaled and can carry photography chemicals into the lungs. Use: Always read and follow the product label instructions. Wear protective gloves, safety goggles, and an organic vapor respirator and cover all exposed skin. Kodak recommends at least 10 air changes per hour for workrooms and recommends exhaust ventilation for the processing and mixing tanks. A canopy-type exhaust hood should be sufficient for photograph development done occasionally in the home; using a bathroom-type exhaust fan is not adequate. Be sure the exhaust fan draws fumes away from you and the work area. Always add acid to water. Never add water to acid when mixing chemical solutions. Avoid products containing Benzene. For information on the ingredients of Kodak products, contact Kodak at 1-800-242-2424 and request a Material Safety Data Sheet. Storage: Keep out of the reach of children and pets. Store acids in nonmetal, unbreakable containers. Store all chemicals in non-breakable containers or place bottles inside plastic containers and clearly mark the contents on the outside. Label the working (diluted) solution with the date it was mixed up in order to avoid using outdated solutions. Disposal: Unmixed chemicals need to be disposed of through a licensed hazardous waste handler or through a professional household hazardous waste collection. It is best to use up your chemicals or check with a school or photographic materials supplier to see if they can use your unwanted supplies to avoid this disposal problem. Properly mixed and diluted black-and-white photography solutions can be flushed with plenty of water down the drain connected to the sanitary sewer system. If you use a septic tank or lagoon, ask a friend or relative who is connected to the sanitary sewer system if you might use their drain to dispose of your properly mixed and diluted photography chemicals. If you have color photography chemicals and solutions contact the manufacturer for disposal instructions. Kodak has a referral number for its products (1-800-242-2424; ask for environmental/technical services).
PIGMENTSA pigment is any substance that gives color to other materials. Pigments which contain Arsenic (A), cadmium (C), or Lead (L) are considered toxic. Common Names of Some Toxic Pigments
PINE OILPine oil is derived from steam distillation of wood from pine trees. It is a common agent used in many household disinfectants and deodorants and has also been used as a pure substance for its disinfectant properties. In general, pine oil in its concentrated form is a skin irritant and may cause allergic reactions. If swallowed, pine oil may be sucked into the lungs (aspirated), possibly resulting in chemical pneumonia.
PLASTIC
GeneralPlastics are materials with a high degree of utility and many safe household uses. They can take the place of other materials that may have more harmful relative impacts. Some plastics, however, are associated with dangers in the manufacturing process, when misused and on other occasions: over their entire life-cycle from manufacture to ultimate disposal. They also comprise a significant fraction o f the entire municipal waste stream. For these reasons the principals of source reduction, reuse and recycling are just as valid for plastics as for other waste products (see Reasonable Responses below). For More current information on solid waste use and recycling patterns in the United States go to:
EPA Municipal Solid Waste Site
General CompositionPlastic is made from fractions of natural gas or crude oil changed chemically into solid form. There are two basic types of plastic: Thermosetting and Thermoplastics. Thermosetting plastics are set to a permanent shape and cannot be softened. These plastics are used primarily for multiple use items, such as dishes and furniture. Thermoplastics are soft when exposed to heat and pressure and harden when cooled. Thermoplastics are the most common type of plastic and are used to make a variety of products. Following is a list of some of the most common types of thermoplastics, along with their recycling code # (the number that appears in the triangle on the bottom), and their common uses.
#1 Polyethylene Terephthalate (PETE) - soft drink containers BEWARE #7! Polycarbonate - This type plastic may contain the suspected endocrine disruptor and carcinogen know as Bisphenol-A. In 1995 the CDC found that Bisphenol-A is likely found in 95% of Americans' blood. This is particularly alarming since evidence is mounting in support of the theory that Bisphenol-A is a possible contributor to the rise in Prostate cancer and other health issues over the last decade. Some examples of products made with polycarbonate plastics are milk containers, water bottles, baby bottles, pacifiers, sippy cups, toys, can liners, food storage containers, dental sealants, plastic eating utensils, and water pipes. No wonder Bisphenol-A is such a common blood contaminant in Americans and why California has already banned many products which may potentially release Bisphenol-A.
General ImpactsWhile plastics have many practical applications and safe household uses, there are also some hazards to plastics. These are the environmental and health impacts throughout the life-cycle of plastics -- from manufacture to use to ultimate disposal.
Plastics are a significant component of the overall waste stream. In 1998 they comprised 10.2% by weight and 24 % by volume (USEPA, Polystyrene Packaging Council). They comprise about 6 percent of all litter. For further information on municipal waste generation and recycling see the US EPA's Waste Information. Plastics do not degrade readily. They never totally biodegrade in the environment because their content is not digestible by microorganisms. If they are not picked up by highway workers or volunteers, then they are moved by air and water or accumulate in low areas, waterways, and along fences. Plastic litter is itself a danger to wildlife. It can kill or injure animals though entanglement in discarded fishing lines, 6-pack rings, and packing bands; or through ingestion of plastic that was mistaken for food.
Toxic Impacts and FDA RoleToxic chemicals are used in the manufacture of plastics. These chemicals include Benzene, cadmium compounds, carbon tetrachloride, chromium oxide, diazomethane, Lead compounds, styrene, and vinyl chloride. Some health studies have shown that people who work in, and live near, plants that manufacture plastics and the chemicals used in plastics experience higher incidents of some kinds of cancer than other population (OSHA).
According to the federal Food and Drug Administration, 55% of all packaging made in the U.S. is for food, with plastics replacing other materials with increasing frequency. While FDA monitors plastics used in food packaging, it has no regulatory authority over plastics with "prior sanction," meaning those that were determined to be safe for use before 1958 (FDA Consumer, 1991). One exception is Ethyl Carbamate (urethane) for which the FDA has prepared an Ethyl Carbamate Preventative Action Manual The most immediate FDA regulatory contact for plastics in food packaging is:
Ms. Hortense L. Macon A recent concern regarding plastic consumer products is connected to phthalates, chemicals used to soften plastics and widely used in plastic toys and children's products. Laboratory experiments have linked phthalates to liver and kidney damage, and tumors. In 1998, the Consumer Product Safety Commission requested that manufacturers remove phthalates from products that children are likely to chew and mouth -- such as nipples, pacifiers, teethers, and soft rattles. Most manufacturers were expected to comply with this request by 1999. To check whether a specific toy contain phthalates, contact the Consumer Product Safety Commission.
Reasonable ResponsesFor the reasons stated previously, it's important to make careful and sparing use of plastics. The familiar pattern of reduce, reuse and recycle is important.
Plastic recycling has grown significantly in quantity and impact. The annual amount of post-consumer plastic has increased at least sixfold since 1990: to 1.45 million pounds in 1998. This is, however still less than 10% of total annual plastic output; it is estimated that at least 80% of all thermoplastics could be melted down and made into new products.
Notes On Incineration and DecompositionIncineration is one way to reduce the volume of plastics headed for the landfill. When burned, plastics release more energy than other municipal wastes. When burned in a municipal incinerator, plastics release more energy than other municipal wastes. However, they also contribute many pollutants, including heavy metals (e.g. cadmium, Lead) to the incinerator's ash and air emissions. The incineration of PVC releases highly corrosive hydrochloric acid, which contributes to acid rain and may lead to the formation of dioxins in the environment. In addition, the energy value of PET plastic bottles is about 11,000 BTUs per pound. However it takes about 49,000 BTUs to produce one pound of PET. So even ignoring potential pollution, burning plastic as fuel is not efficient. We know now that nothing breaks down very quickly in the anaerobic conditions of a landfill, so plastics are expected to remain in landfills unchanged for hundreds of years. There is some evidence of decomposition, however. Phthalates are hazardous substances widely used in plastics manufacture. One of these phthalates (di-2-ethylhexyl phthalate) has been discovered in many leachate samples, leading researchers to believe that the chemicals are leaching from plastics buried in the landfill.
Biodegradable PlasticsPlastics, metals, and glass make up the bulk of the non-organic portion of the municipal solid waste stream. Plastics contribute to the volume of non-organic materials in landfills that are not easily degradable. However, some companies have been researching and have made considerable progress toward the manufacture of plastics using plants as the raw materials, instead of nonrenewable petroleum. These plastics would degrade in a compost environment. In addition, some plastic products have been manufactured to be susceptible to photodegradation (degradation due to sunlight). Photodegradable plastics are primarily used in items like six pack rings that are often littered and may pose a threat to wildlife. The potential for all wastes in landfills to decay may be slower than in the environment. Municipal solid waste landfills today are designed to stay dry in order to reduce the production of leachate and prevent groundwater contamination. There is some pilot testing and research of a different kind of landfill, called a bioreactor. In a bioreactor landfill, liquids are added in order to speed up decomposition so that the landfill will reach stability earlier than in a dry environment. Marie Steinwachs, Office of Waste Management, University of Missouri Outreach and Extension.
POOL CHEMICALSThere are many chemicals used to balance, sanitize, and clean the water in swimming pools (including hot tubs and spas). The following describes the principal chemical products used to maintain pools. Pool water is most comfortable when it is maintained at a pH between 7.2-7.6. The pH scale runs from 1 to 14, where 1-6.9 is acidic, 7 is neutral, and 7.1-14 is alkaline. The pH is controlled by adding either acid or alkali products. An acid, either muriatic acid (also known as hydrochloric acid) or sodium bisulphate, is added to lower the pH. To raise the pH, an alkali, sodium carbonate (also called soda ash) is added. Pools use sanitizing chemicals to remove algae, harmful bacteria, dirt, germs, and organic matter carried into the pool on people's bodies and by the wind. These chemicals oxidize organic matter. Most typically a chlorine product is used to sanitize the pool. Either a liquid or dry product is added; liquid chlorine is usually a 10-15% solution of sodium hypochlorite and dry chlorine is usually calcium hypochlorite. A stabilizer is added to these products to reduce the quantity of chlorine dissipated by the sun. Occasionally, algae will not be controlled by the chlorine sanitizer. A shock treatment of a large amount of chlorine (superchlorination) or a cationic detergent may be added to the water to kill the algae. Alkyl ammonium chlorides are common algaecide ingredients. Many of these pool chemicals are corrosive and are hazardous to human health through skin contact splashed into the eye. Many of these chemicals, including different types of chlorine, can react violently when mixed to produce toxic gases, fire, or explode. Use: Pool chemicals are often in concentrated form and should be handled with thought and care. Carefully read and follow the instructions and warnings on the label of each product. Clearly label the product with the date of purchase. Never mix various pool chemicals together. Use separate and clean scoops for each product: Always add the chemical to the water. Never add water to dry or concentrated chemicals. When adding liquid chlorine into the pool, pour it as far from the pool edge as possible so it will disperse quickly into the water. Pool chemicals should always be added to the pool water separately and according to the time specifications of the directions. Pour chemicals gently into the pool, trying not to splash. Add chemicals while the filter pump is running to aid in rapid dispersal throughout the pool. Do not add chemicals while people are swimming and do not allow swimming again until the chemicals have dispersed for the specified amount of time. Mixing different chlorine products can cause severe reactions or explosions, so if you change brands of solid chlorine, be sure to rinse the dispenser or basket to remove any residue. Do not handle pool chemicals with bare hands or them on eyes, skin, or clothing. Wear protective gloves and safety goggles. Wash hands and all exposed skin after handling chemicals. Do not handle chemicals near sources of ignition as some are flammable. Do not smoke while handling chemicals. Storage: Keep away from children and pet. Close chemical containers tightly. Store them in a clean, dry, and well-ventilated area. Keep liquid and dry chemicals separate. Do not stack pool chemicals. Store pool chemicals away from flammable materials and sources of sparks. Do not store them near metal tools or mechanisms since pool chemicals can cause corrosion. Disposal: The very best way to dispose of these chemicals is to use them up or give them to someone who will. Some suggested places to donate your unwanted pool chemicals are the YMCA, schools, or the park system. If you cannot find an outlet for your chemicals and if you can store them appropriately, secure and hold the chemicals for a professional hazardous waste collection program or give to a licensed hazardous waste handler for disposal. If you cannot find someone to use the unwanted chemicals and you cannot safely store them, flush small quantities down a drain connected to a sewer system with plenty of water. Contact your local wastewater treatment plant to determine if pool chemicals can be flushed into the sanitary sewer system. Be sure to wear protective gloves and safety goggles while doing this. Do not put pool chemicals down the drain if you use a septic tank or lagoon.
RADONRadon (Rn) is a colorless, odorless, radioactive gas. Radon is produced when trace amounts of uranium and radium in the soil or rocks decay. The radon gas will then also decay into radioactive solid particles, called radon daughters or radon progenitors. Some of the short-lived radon daughters attach themselves to small particles in the air, which can be inhaled deep into the lungs. The radon daughters may then damage dividing lung cells, possibly resulting in lung cancer. Radon gas is thought to be responsible for 5,000 to 20,000 lung cancer deaths per year in the United States. For more information about Radon don't miss our complete guide to Radon The major sources of radon are: soil that contains radon-releasing material; water and natural gas that has passed through underground areas containing radon; solar-heating systems that use radon-emitting rocks to store heat; granite rock; and uranium or phosphate mine tailings. Out-of-doors, radon poses little threat to our health because it is in such a low concentration. Indoors, however, radon can become more concentrated because of the lack of ventilation in homes combined with exhaust fans that draw air. Radon gas can seep into a house through dirt floors, cracks in concrete floors and walls, floor drains, sump pumps, and joints. Radon gas can also accumulate in private wells and be released into the home when water is used. This is normally not a problem for large community water supplies. The level of radon that can build up indoors depends upon the amount of radon in the source material and the rate at which it is removed from the home by ventilation. Homes tested throughout the U.S. show a wide range of radon concentrations. Radon is measured in picocuries per liter (pCi/l). One pCi/l means that for each liter of air or water, two radon atoms decay to other atoms per minute. Radon daughters are measured in working levels (WL), which is a gauge of exposure. One WL is approximately equal to 200 pCi/l of radon. The Environmental Protection Agency (EPA) recommends that household levels of radon and radon daughters stay at or below 0.02 WL (= 4 pCi/l). These quick, inexpensive steps advised by the EPA can be taken to help lower your risks from radon exposure:
There are two commercially-available radon detectors; the charcoal canister and the alpha-track detector. Both of these are exposed to the air in your home for a specific time period and sent to a laboratory for analysis. The EPA has put out two informative booklets: "A Citizen's Guide to Radon: What it is and What to do about it", and "Radon Reduction Methods: A Homeowner's Guide." For additional information about radon, contact the American Lung Association.
ROACH KILLERHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
ORGANOPHOSPHATES - Carcinogenic in rats; teratogenic in chick embryos; affect nervous system; acutely toxic causing headache, dizziness, twitching, nausea Bait and trap devices are usually much safer than broadcast or spray pesticide use.
RODENT KILLERHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
WARFARIN - Causes internal bleeding if ingested in large amounts; toxic to fish
SCOURING POWDERMost scouring powders use calcium carbonate as the abrasive, and may contain chlorine bleach. To prevent the formation of toxic gas, do not use cleansers containing bleach with other cleaning products such as toilet bowl cleaner, oven cleaner, or all-purpose cleaners which may contain Ammonia. The combination of bleach and ammonia produces toxic chloramine gas. In sensitive individuals, scouring powder that contains chlorine may irritate and redden the skin. Disposal: Use up as intended or give it to someone who can. If unwanted portions must be disposed of, flush down the drain with plenty of water. However, if on a septic tank or lagoon, dispose of small quantities over a number of days. Alternatives: Baking soda or salt can be easily substituted for scouring powder. Alternative commercial products based upon diatomaceous earth are relatively safe, effective and non-abrasive. Nylon and other non-metallic scrubbing pads may also be effective. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
BLEACH - Fumes highly irritating to eyes and respiratory tract; causes deadly chloramine gas if mixed with ammonia
SEPTIC TANK CLEANERBe wary of products claiming to clean septic tank. Many of these products temporarily precipitate solid giving the illusion of success, but actually they produce solid bulk which is more difficult for bacteria and microorganisms to break down. Many septic tank cleaners also change the water's acidity, causing many bacteria to die, products containing sodium or potassium hydroxide (lye) are highly caustic to humans. They also change the acidity of the water and speed soil clogging. Septic tank cleaners containing organic solvents such as trichloroethylene should never be used because they are toxic, take a very long time to break down in the environment, and have been shown to contaminate the ground water. Cleaners which claim that their "enzymes" will help septic tank digestion have not been found to be effective Bacteria and microorganisms in your septic tank produce their own enzymes and eat only as much as their own enzymes can digest. If your septic tank seems sluggish or overwhelmed, flush a small amount of soil down the drain to replenish the bacteria and microorganisms in your septic tank. Each teaspoon of soil contains hundreds of microorganisms. Use: Avoid septic tank cleaners containing organic solvents. If you are using a septic tank cleaner that contains lye products or sodium bisulfate, wear gloves, goggles, and a respirator with an organic vapor cartridge to avoid fumes and splashes. Storage: Store out of the reach of children. If the cleaner is in a glass jar, store it inside a plastic container and clearly label the outside of the container with the contents. Store on a high shelf or in a locked cabinet. Disposal: Unless the septic tank cleaner is an organic solvent, small amounts of the septic tank cleaner can be flushed down the drain with plenty of water. Organic solvents should be carefully stored until you can dispose of them through a licensed hazardous waste handler or through a professional household hazardous waste collection. Alternatives: Take preventative measures. Avoid putting items down your sink or toilet that bacteria cannot digest or that disrupt their environment. Undigestable items include grease, fat, hair, cigar and cigarette butts, filters, facial tissues, paper towels, napkins, sanitary napkins, and Band-Aids.
SHOE POLISHMany commercial products contain either Trichloroethylene, methylene chloride, or nitrobenzene. These suspected human carcinogens can be easily absorbed through your skin. Use: Wear gloves when polishing or cleaning shoes. After polishing your shoes, be sure they are dry before wearing. Never wear shoes that are not absolutely dry if you are drinking and never drink alcoholic beverages while polishing shoes. The presence of alcohol in the system heightens the toxic effects of nitrobenzene. When toxic quantities of nitrobenzene are absorbed, the person shows a bluish tinge in the fingernail beds, lips, ear lobes, and tongue. Results can be fatal. Unfortunately, most shoe polishes do not list their ingredients. Storage: Keep out of reach of children. Disposal: Use up as intended or give it to someone who will. Take unused portions to a hazardous household waste collection center. If a collection center is unavailable, place it in the trash.
SMOKE DETECTORSSmoke detectors are important for early detection of fires. There are two types of smoke detectors: photoelectric, which detects only visible products of combustion, and ionizing, which detects both the visible and invisible products of combustion. Ionizing (or ion chamber) smoke detectors contain a very small amount of radioactive material, Americium-241 (Am-241). Am-241 has a half life of 458 years and emits alpha particles. The ionizing smoke detector is constructed so that to gain access to the radioactive sections would require the complete destruction of the smoke detector. The best way to dispose of an ionizing smoke detector is to return it to the manufacturer. The photoelectric smoke detector can be disposed of in the trash.
SODIUM CARBONATESodium carbonate, also known as soda ash, washing soda, or sal soda, is white odorless crystals used in the manufacture of soaps, shampoos, bath salts, and some mouthwashes. Sodium carbonate is strongly alkaline and as a dust, it is irritating to the eyes, nose, and upper respiratory tract. Ingestion may produce corrosion of the gastrointestinal tract, vomiting, diarrhea, circulatory collapse, and death. When used in cosmetics it may cause scalp, forehead, and hand rashes.
SODIUM HYPOCHLORITELiquid household bleaches are approximately 5% sodium hypochlorite solutions. Household bleach is an irritant and may cause skin, eye, and respiratory tract irritation. Dermatitis may result from direct skin contact. Ingestion of a few ounces or more of bleach may result in medical complications. Do not mix bleach with acids! Mixing household bleach with acids such as vinegar, Ammonia, toilet bowl cleaners; and drain cleaners produces chloramine gas which can result in burning of mucous membranes and chemical pneumonia. If you use "fresh scented" bleach be aware that it may mask your natural ability to nasally detect overexposure to the bleach product.
SOLVENTSA solvent is any substance that dissolves another substance. For example, mineral spirits (a petroleum solvent) dissolves paint. Water, the most common solvent, is an "inorganic" solvent because it does not contain carbon. Many solvents used in the home are "organic" solvents. All organic solvents are hazardous! Organic solvents used in household products are hazardous and contain the same ingredients as their industrial and commercial counterparts. Solvents are used in many household products. Products containing almost 100% solvents include paint thinner, furniture stripper, dry-cleaning fluid, spot remover, degreaser, turpentine, and nail polish remover. Products that are composed partially of solvents include furniture oil, glues, aerosol sprays, shoe care products, rug cleaners, and oil-based paints. Solvents can be flammable, toxic, or pose a serious health risk through skin absorption and inhalation. Some health hazards occur immediately. Others, such as liver and kidney problems, birth defects, and nervous disorders, occur slowly over time. If absorbed through the skin, solvents are readily passed into the bloodstream where they must be filtered out by the liver and kidneys, the body's first line of defense against toxins. Solvent vapors are easily inhaled. Inhalation may cause nose and throat irritation and damage to lung tissue. Solvent vapors or splashes can cause severe eye damage, especially to those wearing soft contact lenses because the lenses absorb the solvents and hold them next to the eye. In addition, many solvents adversely affect the central nervous system, producing drunken or narcotic effects which can permanently affect normal functions. Intentional inhalation of solvents can result in unconsciousness and death. Some Toxic Organic Solvents common to Household Products:
Environmental concerns are also associated with solvents. During normal use, solvents escape into the environment where they contribute to smog-producing air pollutants. If disposed of improperly, solvents can contribute to groundwater pollution. Use: To protect yourself from the ill effects of solvents; work in a well-ventilated area and use a fan to direct fumes away from the area where you are working and to the out-of-doors. Wear goggles, gloves, and clothing that covers exposed skin. After handling solvents, always wash your hands and any exposed skin before eating or smoking. Do not drink alcoholic beverages while using solvents because they intensify the toxic effects. Storage: solvents should be stored in a well-ventilated area, away from children and pets. If the solvent product is flammable or contained in an aerosol spray, precautions should also be taken to store away from heat, flame, or sources of ignition. Disposal: The best way to dispose of solvents is to use them up as intended. If you have usable solvent and cannot use it yourself, donate your leftovers to someone who will use it up. Otherwise, solvents should be safely secured and stored for a professional household hazardous waste collection or taken to a licensed hazardous waste handler.
"Spot-ons" FLEA and TICK SPRAY
What is it? A "Spot-on" is an insecticide product that is named for the way it is applied. It's applied to a small area or "spot," on your dog or cat. It comes in liquid form. The pesticide in the "Spot-on" works by "spreading out" over your pet to kill and repel fleas and ticks. Remember to always read the label first to know how to properly use these products and for safety information.
What's in it?
What health and safety things do you need to think about with "Spot-ons?
SPOT REMOVERHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
PERCHLOROETHYLENE - Fumes are carcinogenic and acutely toxic, cause dizziness, sleepiness, nausea, loss of appetite and disorientation
STARCHHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
Formaldehyde - A suspected carcinogen and a strong irritant to the eyes, throat, skin and lungs
SULFUR DIOXIDESulfur dioxide is a by-product of the burning (combustion) of fossil fuels, which include coal, petroleum, kerosine, propane and oil. Vision difficulty and irritability as well as diminished breathing capacity and worsening of lung and heart disease may result from sulphur dioxide fumes. If you use any appliances that burn fossil fuels, make certain that there is adequate ventilation in the room. For example, open a window a crack for fresh air. Sulfur dioxide contributes to acid rain.
SULFURIC ACIDSulfuric acid, also known as oil of vitriol, hydrogen sulfate, or spirit of sulfur, is available in powder form and as a colorless, odorless, oily liquid. Beware - it is a highly corrosive liquid! Sulfuric acid is used as an electrolyte in wet cell batteries and as an ingredient in toilet bowl cleaners (sodium bisulfate). Direct contact can cause burning and charring of the skin and causes rapid injury to the mucous membranes. It is exceedingly dangerous to the eyes. Exposure to sulfuric acid mist and subsequent inhalation causes irritation of the respiratory tract and mucous membranes including the eyes. The mist also causes etching of tooth enamel. Ingestion results in serious burns to the mouth, esophagus, and stomach. Even dilute sulfuric acid can irritate the skin and mucous membranes and cause scarring of the face and eyelids and irreparable damage to the cornea, resulting in blindness.
TOILET CLEANERSHAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
SODIUM BISULFATE - Forms sulfuric acid, which is corrosive, burns skin
TOLUENE & XYLENEToluene (also known as toluol or methylbenzene) and xylene (also known as xylol or dimethylbenzene) are aromatic hydrocarbons found around the home in paints, paint and varnish removers, degreasers, cleaners, lacquers, glues, nail polish, and cement. Because of their excellent ability to dissolve substances, they are often used in insecticides and other pesticides to dissolve the active ingredient. Toluene and xylene are volatile, flammable, and toxic. Toluene and xylene are irritating to the skin and respiratory tract and may cause liver damage. These aromatic hydrocarbons enter your system through inhalation and ingestion, but are poorly absorbed by the skin. The target organs attacked by toluene and xylene are the central nervous system, eyes, liver, kidneys, and skin. Toluene and xylene are narcotic in high concentrations. Intentional inhaling of these substances can cause headache, giddiness, and a transient euphoria followed by depression. Hallucinations may occur, especially following chronic exposure. Neurological damage occurs from concentrated inhalation of these fumes. Symptoms include fatigue, weakness, confusion, headache, tearing, nervousness, muscular fatigue, insomnia, dermatitis, an intolerance of light. For an extensive collection of facts about Xylene, including specific household product sources, health effects, safe handling, and toxicological information, you may want to visit our Complete Guide to Xylene. You may also be interested in our Complete Guide to Toluene which includes material safety data sheet information for Toluene plus specific household chemical sources of Toluene, health statement, FAQs, and tips for safe Toluene use.
TRICHLOROETHANETrichloroethane is a colorless, nonflammable liquid with a pungent odor similar to chloroform. It is commonly used as a solvent and cleaning agent in spot removers and fabric cleaners, film cleaner, insecticides, paint and varnish remover, degreaser, typewriter correction fluid, and as an aerosol propellant. Trichloroethane is absorbed by inhalation and ingestion. It is an irritant to the eyes and nose and can result in central nervous depression and liver and kidney damage if ingested.
TRICHLOROETHYLENETrichloroethylene is a colorless liquid with a sweet chloroform-like odor. It can be found in septic tank cleaners and degreasers as a solvent. Trichloroethylene is persistent, toxic, and mobile. Its use has been shown to contaminate the groundwater; its use should be avoided in septic tank compounds.
WEED KILLER
What is it? Weed killing pesticides come in different forms – sprays, powders, or mixed in with fertilizers. They are also known as herbicides. So how does a weed killing herbicide know what is a weed and what isn't? It doesn't. For example, a herbicide that is formulated or made to kill bushy weeds like poison ivy or poison oak, can also kill other plants, grasses and even trees. That's why it is important to always read the label first.
What's in it?
What health and safety things do you need to think about with weed killers?
WINDOW & GLASS CLEANERSWindow and glass cleaner commonly contains isopropyl alcohol or Ammonia, water, and coloring. It may be mildly irritating to the eyes, skin, nose, and throat. Use: Always use window and glass cleaners in a well-ventilated area. Storage: Keep out of reach of children. Disposal: Unused or unwanted portions of window or glass cleaner should be flushed down the drain with plenty of water. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
AMMONIA - Fumes irritate eyes, lungs; can cause burns or rashes on skin
WINDSHIELD WIPER SOLUTIONWindshield wiper solution contains methanol (37%-100%), detergent, and water. Due to its hazardous nature, windshield wiper solution is required to have a child-proof safety cap. The most toxic windshield wiper solutions contain 100% methanol. For health and environmental effects associated with this products please refer to methanol. Use: Avoid skin contact and inhalation. Wear gloves when adding windshield wiper fluid to your car. Storage: Store away from children and pets. Make sure safety cap is on securely. Disposal: If the windshield wiper solvent contains methanol the product should be disposed of by a licensed hazardous waste handler or through a household hazardous waste collection. To avoid this disposal dilemma, it is best to get rid of this product by using it up as intended. To dispose of unwanted fluid that does not contain methanol, small quantities can be flushed down the drain with plenty of water if your drain is hooked up to a sanitary sewer system. If you use a septic tank or lagoon, ask a friend, relative, or neighbor to use their drain to dispose of your unwanted windshield wiper solvent. Disposal of this product down a drain connected to the septic system can overwhelm the microorganisms and result in damage to the system. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
METHANOL - Damages the nervous system, liver, kidneys, inhalation can lead to lung disease, ingestion can cause blindness
WOOD PRESERVATIVESWood preservatives are products containing pesticides which protect wood from pests and rot. Three widely used wood preservatives - creosote, inorganic Arsenic compounds (CCA), and pentachlorophenol (penta) are highly toxic. In November 1986, the use of wood preservatives containing these compounds was restricted, which means that only licensed applicators can now purchase products containing these compounds. Creosote and inorganic arsenic compounds have been shown to cause cancer in humans, pentachlorophenol in lab animals. In addition, creosote has been linked to genetic damage, inorganic arsenic compounds are related to both genetic damage and birth defects, and pentachlorophenol is associated with birth defects and fetal toxicity. The Environmental Protection Agency strongly suggests that homeowners leave work involving wood preservatives to professionals. If you have a deck, tables, or other furniture that are treated with one of these types of wood preservatives, EPA advises sealing it with at least two coats of shellac or other sealant. Currently when creosote or pentachlorophenol is used on wood intended for human contact, it must be coated with shellac or another sealant. Use: Never burn treated wood in the fireplace; the fumes will be toxic. Special training is required to learn the proper precautions for applying wood preservatives that contain creosote, inorganic arsenic compounds, or pentachlorophenol. Storage: Keep out of the reach of children. Store in well-ventilated area in a box lined with plastic bags. Carefully label the outside of the box with its contents. Disposal: Wood preservatives that contain creosote inorganic arsenic compounds, or pentachlorophenol need to be disposed of by a licensed hazardous waste handler or through a professional household hazardous waste collection. There is no good means to safely dispose of leftover wood preservative short of getting rid of it by using it up as it was intended. HAZARDOUS HOUSEHOLD CHEMICAL INGREDIENTS and Possible Effects:
PENTACHLOROPHENOL - Toxic to fetus and causes birth defects, toxic if inhaled, absorbed, or ingested
WOOD STAINS AND FINISHES
What is it?
What's in it?
What health and safety precautions do you need to think about with stains/finishes?
The average house contains thousands of indoor air pollutants which consist of biological pollutants (such as viruses, bacteria, and fungi), chemical gases such as Radon, and other hazardous substances from numerous household products. This page lists ingredients and hazards for over 100 Household products which are a major source of these Volatile Organic Chemicals. Particles and residues also result from these household products and many other chemical sources that are part of your home's structure and contents. An effective home air purifier and ventilation are two of the three main ways to improve indoor air quality. However, source control (the third primary method) is grossly neglected by most home owners particularly with regards to household chemical toxins.
Many of the household chemicals and products listed in the chart below are not needed; even worse, the use of some of them result in health and environmental costs which far outweigh their benefits. But guess what?..most of us have most of those products in our homes and maybe a couple hundred more. Bottles, cans, boxes, and bags of household chemicals are carelessly stockpiled in our crawlspaces, basements, attics, bathrooms, garages, kitchens, tool sheds, laundry rooms, bedrooms, and closets. It's chemical "over kill", literally. When one becomes aware of the toxic ingredients in many common household products it should be a "no-brainer" to determine that the cost to benefit of using and manufacturing them is highly detrimental. Considering the resultant hazardous waste generation (such as when household chemicals are manufactured or disposed of) it seems obvious that solving one problem (such as stripping paint or cleaning the oven) often begets worse problems, no matter how gradual, chronic, or belated they might be. Nevertheless, most people assume the government will protect them and thus mistakenly think that as a result household chemicals are more benign or safer than industrial chemicals. THEY ARE WRONG! An "out of sight, out of mind" mentality about the resultant industrial pollution and health risks is dangerous given that household chemicals and industrial chemicals are often one in the same as far as risk to environment, health, and safety are concerned. Given this misunderstanding it is no wonder our homes are filled with thousands of the same toxic substances that strongly correlate to lung disease, cancer, and many other chronic diseases...Why?...Well it all goes back to marketing and education.
GALLAXY GLUE...GALLAXY GLUE...THE WORLD WOULD GO TO PIECES WITHOUT GALLAXY GLUE!!!"
At the same time consumers' apathy about chemical dangers is taken advantage of by corporations using potent chemical ingredients as tools to create an ever more complex line of marketable household products to fullfill demand bred of our "quick-fix" mentalities. We are literally being cross-merchandised to death with regards to household chemical sales. Corporate marketing has been very successful at "brainwashing" consumers into believing that they need a specific product for a specific job. Buying a specific household product to clean the toilet or shower or oven or automobile or deck is ludicrous when in fact one product may work for most cleaning jobs throughout the house. There are hundreds of massive corporations pumping out millions of tons of toxic household chemicals branded into tens of thousands of products that have infiltrated most homes across the US to an alarming degree. How many branded toxic "trade secrets" are in your cabinets? Unfortunately for the ecosystem, our health, and future generations' health, under the current state of affairs most of us will remain "chemical Hobgoblins". We will continue to slowly but surely poison ourselves at every turn. The scientists and government agencies know that Cancer is now an epidemic, with 1 in 2 men and 1 in 3 women likely to get some form of cancer at some point in their lifetimes. Asthma and allergy cases have also mushroomed in the last 20 years, making for an epidemic of their own. Part of the reason is no-doubt because we are bombarded with chemicals from all fronts - food, water, and air. It's too bad we as a species are typically REACTIONARY rather than PROACTIONARY, unless of course there arises a capital force to make pre-emption profitable. Usually we end up learning the hard way via our mistakes; trial and error as they say. We're in that phase right now with regards to pollution, indoor and otherwise.
Even as levels of hundreds of toxic man-made chemicals (many of the same ones discussed below) continue to rise in each and every one of our bloodstreams, autism quintuples, cancer marches on, and birth-defects mount - the corporate marketing machines are spinning out their propaganda harder than ever. They wouldn't want the ugly truth getting in the way of their herds of cash cows enthusiastically and conspicuously consuming the "coolaid", now would they? (I hope you can detect my sarcasm here?) So why are our bloodstreams contaminated with toxic chemicals? Well let's look at where you spend most of your time and where most of the chemical sources are that we come in contact with via air, food, or water - yes, you guessed it, YOUR HOME! Most of our humble abodes are extremely over-loaded with hazardous household chemicals (to the delight of chemical manufacturers), most of which perpetually "leak" volatile gases and other nasty residues into your indoor air. Many of these household chemical products contain the very same toxic waste components found in the majority of Superfund waste sites (and that's no coincidence).
To get you started on your journey toward eliminating sources of chemical indoor air pollutants in your home we compiled the below chart which has over 100 of the most common household chemical sources. First, identify the problems in your home using the below chart. Then, once you've learned of all the dangerous household chemicals in your home, you may want to discover how you can eliminate some of them completely (where practical). Following is a link to my frugal living website which has a large list of my favorite natural home made household products such as my environmentally friendly cleaning products. I personally use many of these green cleaning products because they are cheap, safe, and effective. Don't be just another wasteful chemical consuming Hobgoblin. Start by going green with this extensive collection of green cleaning products and many other Home made Household Products for safe and frugal living. That's one of the most extensive lists you will find on the internet for quick and easy recipes for making your own non-toxic home-made cleaners and other homemade products. These home-brew household cleaners are CHEAP, SIMPLE, NATURAL, SAFE, AND EFFECTIVE.
Make your own. Save money. Save the planet. And save your health. Let's kick those corporate chemical cattle drivers to the curve!
We hope you found this household chemical guide helpful in your journey toward creating a health home environment. We will be updating this page and site regularly with new information about the most common household chemical hazards including tips on safe usage, storage, and disposal. Also in development is more resources for helping consumers kick those corporate fat cats bearing toxic chemical gift horses out of your home. So look for more household chemical alternatives (such as homemade cleaners and home-made disinfectants) which may be much cheaper, more effective, natural, non-toxic, and non-hazardous. |









