This public health statement tells you about formaldehyde and the effects of exposure.
The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites make up the National Priorities List (NPL) and are the sites targeted for long-term federal cleanup activities. Formaldehyde has been found in at least 26 of the 1,428 current or former NPL sites. However, it's unknown how many NPL sites have been evaluated for this substance. As more sites are evaluated, the sites with formaldehyde may increase. This is important because exposure to this substance may harm you and because these sites may be sources of exposure.
When a substance is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. This release does not always lead to exposure. You are exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking the substance or by skin contact.
If you are exposed to formaldehyde, many factors determine whether you'll be harmed. These factors include the dose (how much), the duration (how long), and how you come in contact with it. You must also consider the other chemicals you're exposed to and your age, sex, diet, family traits, lifestyle, and state of health.
Formaldehyde can react with many other chemicals, and it will break down into methanol (wood alcohol) and carbon monoxide at very high temperatures.
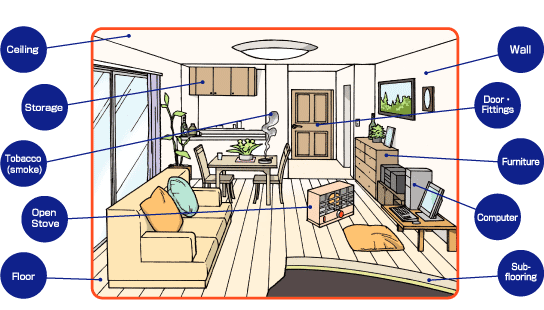
Formaldehyde is naturally produced in very small amounts in our bodies as a part of our normal, everyday metabolism and causes us no harm. It can also be found in the air that we breathe at home and at work, in the food we eat, and in some products that we put on our skin. A major source of formaldehyde that we breathe every day is found in smog in the lower atmosphere. Automobile exhaust from cars without catalytic converters or those using oxygenated gasoline also contain formaldehyde. At home, formaldehyde is produced by cigarettes and other tobacco products, gas cookers, and open fireplaces. It is also used as a preservative in some foods, such as some types of Italian cheeses, dried foods, and fish. Formaldehyde is found in many products used every day around the house, such as antiseptics, medicines, cosmetics, dish-washing liquids, fabric softeners, shoe-care agents, carpet cleaners, glues and adhesives, lacquers, paper, plastics, and some types of wood products. Some people are exposed to higher levels of formaldehyde if they live in a new mobile home, as formaldehyde is given off as a gas from the manufactured wood products used in these homes.
Following Hurricane Katrina, many families filed complaints with FEMA due to respiratory illness and symptoms of Sick Building Syndrome in the FEMA trailer temporary housing. Their symptoms have been linked to Formaldehyde off-gasing from building materials used in the manufacture of the FEMA trailers the Katrina evacuees were being temporarily housed in.
Elevated levels of Formaldhyde in FEMA trailers is proof that materials such as particle board and carpeting can be hazardous to your health due to chemical off-gasing. It is unknown how many lawsuits and toxic tort cases related to Formaldehyde exposure are pending - but Formaldehyde IS known to be a common source of Sick Building Syndrome.
Formaldehyde is used in many industries. It is used in the production of fertilizer, paper, plywood, and urea-formaldehyde resins. It is present in the air in iron foundries. It is also used in the production of cosmetics and sugar, in well-drilling fluids, in agriculture as a preservative for grains and seed dressings, in the rubber industry in the production of latex, in leather tanning, in wood preservation, and in photographic film production. Formaldehyde is combined with methanol and buffers to make embalming fluid. Formaldehyde is also used in many hospitals and laboratories to preserve tissue specimens.
There is usually more formaldehyde present indoors than outdoors. Formaldehyde is released to the air from many home products and you may breath in formaldehyde while using these products. Latex paint, fingernail hardener, and fingernail polish release a large amount of formaldehyde to the air. Plywood and particle board, as well as furniture and cabinets made from them, fiberglass products, new carpets, decorative laminates, and some permanent press fabrics give off a moderate amount of formaldehyde. Some paper products, such as grocery bags and paper towels, give off small amounts of formaldehyde. Because these products contain formaldehyde, you may also be exposed on the skin by touching or coming in direct contact with them. You may also be exposed to small amounts of formaldehyde in the food you eat. You are not likely to be exposed to formaldehyde in the water you drink because it does not last a long time in water.
Many other home products contain and give off formaldehyde although the amount has not been carefully measured. These products include household cleaners, carpet cleaners, disinfectants, cosmetics, medicines, fabric softeners, glues, lacquers, and antiseptics. You may also breathe formaldehyde if you use unvented gas or kerosene heaters indoors or if you or someone else smokes a cigar, cigarette, or pipe indoors. The amount of formaldehyde in mobile homes is usually higher than it is in conventional homes because of their lower air turnover.
People who work at or near chemical plants that make or use formaldehyde can be exposed to higher than normal amounts of formaldehyde. Doctors, nurses, dentists, veterinarians, pathologists, embalmers, workers in the clothing industry or in furniture factories, and teachers and students who handle preserved specimens in laboratories also might be exposed to higher amounts of formaldehyde. The National Institute for Occupational Safety and Health (NIOSH) estimates that 1,329,332 individuals in the United States have had the potential for occupational exposure to formaldehyde.
To protect the public from the harmful effects of toxic chemicals and to find ways to treat people who have been harmed, scientists use many tests.
One way to see if a chemical will hurt people is to learn how the chemical is absorbed, used, and released by the body; for some chemicals, animal testing may be necessary. Animal testing may also be used to identify health effects such as cancer or birth defects. Without laboratory animals, scientists would lose a basic method to get information needed to make wise decisions to protect public health. Scientists have the responsibility to treat research animals with care and compassion. Laws today protect the welfare of research animals, and scientists must comply with strict animal care guidelines.
Several studies of laboratory rats exposed for life to high amounts of formaldehyde in air found that the rats developed nose cancer. Some studies of humans exposed to lower amounts of formaldehyde in workplace air found more cases of cancer of the nose and throat (nasopharyngeal cancer) than expected, but other studies have not found nasopharyngeal cancer in other groups of workers exposed to formaldehyde in air. The Department of Health and Human Services (DHHS) has determined that formaldehyde may reasonably be anticipated to be a human carcinogen (NTP). The International Agency for Research on Cancer (IARC) has determined that formaldehyde is probably carcinogenic to humans. This determination was based on specific judgments that there is limited evidence in humans and sufficient evidence in laboratory animals that formaldehyde can cause cancer. The Environmental Protection Agency (EPA) has determined that formaldehyde is a probable human carcinogen based on limited evidence in humans and sufficient evidence in laboratory animals.
Formaldehyde is likely to contribute to Occupational Asthma and Allergic Contact Dermititis
Children and adults are likely to be exposed to formaldehyde in the same way. The most common way for children to be exposed to formaldehyde is by breathing it. Children may also be exposed by wearing some types of new clothes or cosmetics. A small number of studies have looked at the health effects of formaldehyde in children. It is very likely that breathing formaldehyde will result in nose and eye irritation (burning feeling, itchy, tearing, and sore throat). We do not know if the irritation would occur at lower concentrations in children than in adults. Studies in animals suggest that formaldehyde will not cause birth defects in humans. Inhaled formaldehyde or formaldehyde applied to the skin is not likely to be transferred from mother to child in breast milk or to reach the developing fetus.
Formaldehyde is usually found in the air. Formaldehyde levels are also higher indoors than outdoors. Opening windows or using a fan to bring in fresh air is the easiest way to lower formaldehyde levels in the home and reduce the risk of exposure to your family.
Removing formaldehyde sources from the house will also reduce the risk of exposure. Since formaldehyde is found in tobacco smoke, not smoking or smoking outside will reduce exposure to formaldehyde. Unvented heaters, such as portable kerosene heaters, also produce formaldehyde. If you do not use these heaters in your home or shop, you help to prevent the build up of formaldehyde indoors.
Formaldehyde is found in small amounts in many consumer products including antiseptics, medicines, dish-washing liquids, fabric softeners, shoe-care agents, carpet cleaners, glues, adhesives, and lacquers. If you or a member of your family uses these products, providing fresh outdoor air when you use them. This will reduce your exposure to formaldehyde. Some cosmetics, such as nail hardeners, have very high levels of formaldehyde. If you do not use these products in a small room, or if you have plenty of ventilation when you use them, you will reduce your exposure to formaldehyde. If your children are not in the room when you use these products, you will also reduce their exposure to formaldehyde.
Formaldehyde is emitted from some wood products such as plywood and particle board, especially when they are new. The amount of formaldehyde released from them decreases slowly over a few months. If you put these materials in your house, or buy furniture or cabinets made from them, opening a window will lower formaldehyde in the house. The amount of formaldehyde emitted to the house will be less if the wood product is covered with plastic laminate or coated on all sides. If it is not, sealing the unfinished sides will help to lower the amount of formaldehyde that is given off.
Some permanent press fabrics emit formaldehyde. Washing these new clothes before use will usually lower the amount of formaldehyde and reduce your family's risk of exposure.
Regulations and recommendations can be expressed in not-to-exceed levels in air, water, soil, or food that are usually based on levels that affect animals, then they are adjusted to help protect people. Sometimes these not-to-exceed levels differ among federal organizations because of different exposure times (an 8-hour workday or a 24-hour day), the use of different animal studies, or other factors.
Recommendations and regulations are also periodically updated as more information becomes available. For the most current information, check with the federal agency or organization that provides it. Some regulations and recommendations for formaldehyde include the following:
Several international, national, and state authorities have established regulations or guidelines for the use and production of formaldehyde. OSHA has established the permissible exposure limit (PEL) 8-hour time-weighted average (TWA) at 0.75 ppm and the 15-minute Short-Term Exposure Limit (STEL) at 2 ppm. The EPA sets regulations for reporting quantities used and how much formaldehyde can legally be produced from automobile exhaust; the FDA also has regulations about the use of formaldehyde in the food you eat.
Non-enforceable guidelines have also been established for formaldehyde. The American Conference of Governmental and Industrial Hygienists (ACGIH) has established a ceiling limit for occupational exposure (Threshold Limit Value [TLV]) of 0.4 ppm. NIOSH has a recommended exposure limit for occupational exposure (8-hour TWA) of 0.016 ppm, and a 15-minute ceiling limit of 0.1 ppm.
If you have any more questions or concerns, please contact your community or state health or environmental quality department or:
Agency for Toxic Substances and Disease Registry Division of Toxicology1600 Clifton Road NE, Mailstop F-32Atlanta, GA 30333
Phone: 888-422-8737 FAX: (770)-488-4178ATSDR can also tell you the location of occupational and environmental health clinics. These clinics specialize in recognizing, evaluating, and treating illnesses resulting from exposure to hazardous substances.
Synonyms and IdentifiersHuman Health Effects:
Evidence for Carcinogenicity:
CLASSIFICATION: B1; probable human carcinogen. BASIS FOR CLASSIFICATION: Based on limited evidence in humans, and sufficient evidence in animals. Human data include nine studies that show statistically significant associations between site-specific respiratory neoplasms and exposure to formaldehyde or formaldehyde-containing products. An increased incidence of nasal squamous cell carcinomas was observed in long-term inhalation studies in rats and in mice. The classification is supported by in vitro genotoxicity data and formaldehyde's structural relationships to other carcinogenic aldehydes such as acetaldehyde. HUMAN CARCINOGENICITY DATA: Limited. ANIMAL CARCINOGENICITY DATA: Sufficient.
[U.S. Environmental Protection Agency's Integrated Risk Information System (IRIS) on Formaldehyde (50-00-0) Available from: http://www.epa.gov/ngispgm3/iris on the Substance File List as of March 15, 2000]**PEER REVIEWED**
A2. A2= Suspected human carcinogen.
[American Conference of Governmental Industrial Hygienists. Threshold Limit Values (TLVs) for Chemical Substances and Physical Agents and Biological Exposure Indices (BEIs) for 1995-1996. Cincinnati, OH: ACGIH, 1995., p. 22]**PEER REVIEWED**
Evaluation: There is limited evidence in humans for the carcinogenicity of formaldehyde. There is sufficient evidence in experimental animals for the carcinogenicity of formaldehyde. Overall evaluation: Formaldehyde is probably carcinogenic to humans (Group 2A).
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V62 336 (1995)]**PEER REVIEWED**
Human Toxicity Excerpts:
IF SOLN IS INGESTED, MUCOUS MEMBRANES OF MOUTH, THROAT, & INTESTINAL TRACT ARE IRRITATED, & SEVERE PAIN, VOMITING, & DIARRHEA RESULT. AFTER ABSORPTION, FORMALDEHYDE DEPRESSES CNS & SYMPTOMS NOT UNLIKE THOSE OF ALC INTOXICATION ARE NOTED. THEY CONSIST OF VERTIGO, DEPRESSION, & COMA. RARELY CONVULSIONS ARE OBSERVED.
[Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 993]**PEER REVIEWED**
ALTERATION OF TISSUE PROTEINS BY FORMALDEHYDE CAUSES LOCAL TOXICITY & PROMOTES ALLERGIC REACTIONS. REPEATED CONTACT WITH SOLN ... MAY CAUSE ECZEMATOID DERMATITIS. DERMATITIS FROM CLOTHING TREATED WITH FORMALDEHYDE ... HAS OCCURRED.
[Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 971]**PEER REVIEWED**
AQ SOLN ... SPLASHED OR DROPPED ON HUMAN EYES HAVE CAUSED INJURIES RANGING FROM SEVERE PERMANENT CORNEAL OPACIFICATION & LOSS OF VISION TO MINOR TRANSIENT INJURY OR DISCOMFORT, DEPENDING UPON WHETHER SOLN WERE OF HIGH OR LOW CONCN.
[Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 443]**PEER REVIEWED**
INHALATION OF HIGH CONCN ... CAUSED SEVERE IRRITATION OF RESP TRACT, LEADING IN 2 INSTANCES TO DEATH. ... PULMONARY EDEMA, WITH RESIDUAL CARDIAC IMPAIRMENT IN 1 CASE, WAS REPORTEDLY CAUSED BY SINGLE ACUTE INHALATIONS ... .
[American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices. 5th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1986., p. 276]**PEER REVIEWED**
IN SENSITIZED SUBJECTS SPECIFIC LATE ASTHMATIC REACTIONS MAY BE PROVOKED BY BRIEF EXPOSURES AT APPROX 3 PPM.
[HENDRICK DJ ET AL; J OCCUP MED 24 (11): 893 (1982)]**PEER REVIEWED**
Ingestion of formaldehyde can cause a reduction in body temperature.
[Environment Canada; Tech Info for Problem Spills: Formaldehyde p.83 (1985)]**PEER REVIEWED**
Symptoms related to ingestion of formaldehyde include: jaundice, acidosis, & hematuria. Symptoms related to inhalation include: rhinitis, anosmia, laryngospasm, tracheitis, & gastroenteritis.
[ITII. Toxic and Hazardous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute, 1988., p. 250]**PEER REVIEWED**
In a survey of 57 embalmers who were exposed to atmospheric concn below 2 ppm, there was a high incidence of symptoms of irritant effects on the eyes (81%) nose & throat (75%). Other respiratory effects included cough (33%), chest tightness (23%), wheezing (12%), & shortness of breath (11%). On the basis of the results, 10% were acute bronchitics, & 30% were chronic bronchitics. No control group was used & cigarette smoking was not taken into account.
[Plunkett ER, Barbela T; Am Ind Hyg Assoc J 38: 61 (1977)]**PEER REVIEWED**
Eyes: concn 1-10 ppm may produce appreciable eye irritation on initial exposure; lacrimation occurs at about 4 ppm.
[Health and Safety Executive Monograph: Formaldehyde p.8 (1981)]**PEER REVIEWED**
CULTURED BRONCHIAL & FIBROBLASTIC CELLS FROM HUMANS WERE USED TO STUDY DNA DAMAGE & TOXICITY. FORMATION OF CROSSLINKS BETWEEN DNA & PROTEINS, CAUSED SINGLE-STRAND BREAKS IN DNA, & INHIBITED RESEALING OF SINGLE-STRAND BREAKS PRODUCED BY IONIZING RADIATION.
[GRAFSTROM RC ET AL; SCIENCE 220 (4593): 216-8 (1983)]**PEER REVIEWED**
Formaldehyde induced a 1.5-3 fold increase in sister chromatid exchanges in ... human lymphocytes in culture.
[Obe G, Beek B; Drug and Alcohol Dependence 4: 91-4 (1979)]**PEER REVIEWED**
Formaldehyde was mutagenic for diploid human lymphoblasts in culture ... /inducing an incr number of mutations at/ 130 uM or 4 ppm by weight.
[Goldmacher VS et al; Toxicol Epidemiol Mech (Pap Meet) 173-91 (1983)]**PEER REVIEWED**
OUTBREAK OF HEMOLYTIC ANEMIA, ATTRIBUTED TO ACCIDENTAL EXPOSURE ... OCCURRED AMONG PATIENTS ON HEMODIALYSIS. 41 YR OLD WOMAN DIED 28 HR AFTER INGESTING 120 ML OF ... SOLN (37% WT/VOL FORMALDEHYDE, 12.5% VOL/VOL METHANOL, CONTAINING NO FORMIC ACID).
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V29 369 (1982)]**PEER REVIEWED**
EFFECTS IN WOMEN ATTRIBUTED TO EXPOSURE ... INCL MENSTRUAL DISORDERS & SECONDARY STERILITY.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V29 370 (1982)]**PEER REVIEWED**
SYMPTOMATOLOGY: A. Inhalation: 1. Irritation of mucous membranes, especially of eyes, nose & upper respiratory tract. 2. With higher concn, cough, dysphagia, bronchitis, pneumonia, edema or spasm of the larynx. Pulmonary edema is uncommon. B. Ingestion. 1. Immediate intense pain in mouth, pharynx & stomach. 2. Nausea, vomiting, hematemesis, abdominal pain & occasionally diarrhea (which may be bloody). 3. Pale, clammy skin & other signs of shock. 4. Difficult micturition, hematuria, anuria. 5. Vertigo, convulsions, stupor, & coma. 6. Death due to respiratory failure. C. Skin contact: 1. Irritation & hardening of skin. Strong solutions produce coagulation necrosis. 2. Dermatitis & hypersensitivity from prolonged or repeated exposure.
[Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. III-197]**PEER REVIEWED**
INVESTIGATIONS OF CILIOSTATIC EFFECT OF ALDEHYDES ARE OF SPECIAL INTEREST SINCE MANY HAVE IRRITATING EFFECT ON TRACHEAL MUCOSA. COMPARISON OF CILIOSTATIC EFFECT SHOWED FORMALDEHYDE TO BE MOST TOXIC FOLLOWED BY ACETALDEHYDE & ACROLEIN. CROTONALDEHYDE & METHACROLEIN SHOWED WEAKEST EFFECT. TECHNIQUE USED FOR OBSERVING TRACHEAL CILIARY ACTIVITY WAS THE IN VITRO TECHNIQUE.
[DALHAMN T, ROSENGREN A; ARCH OTOLARYNGOL 93 (5): 496-500 (1971)]**PEER REVIEWED**
One hundred nine workers & 254 control subjects were studied to evaluate the effects of formaldehyde on the mucous membranes & lungs. A modified, respiratory symptom questionnaire & spirometry were admin to all study participants before & after their work shift, & formaldehyde levels were determined for each test subject. Over the course of the monitored work shift, test subjects demonstrated a dose-dependent excess of irritant symptoms & a statistically significant decline in certain lung function parameters. Baseline spirometry values were not significantly different between test & control groups, & formaldehyde-exposed workers did not report an excess of respiratory symptoms. Formaldehyde is a dose-dependent irritant of the eyes & mucous membranes at low-level exposures. It can exert a small, across-shift effect on airways but after a mean exposure of 10 yr does not appear to cause permanent respiratory impairment.
[Horvath EP et al; J Am Med Assoc 259 (5): 701-7 (1988)]**PEER REVIEWED**
The effect of formaldehyde exposure on medical students conducting dissections in the gross anatomy laboratory course /was evaluated using/ self-administered questionnaires designed to assess the frequency of occurrence of various symptoms indicating the acute effects of formaldehyde exposure. The questionnaires were given to a cohort of 1st-yr medical students on completion of the gross anatomy lab course. Air sampling of formaldehyde levels in the anatomy labs was carried out on one day during the time in which these students were conducting dissections. ... Although the results of the air sampling showed formaldehyde levels to be well below current occupational standards, significant numbers of students reported experiencing symptoms associated with formaldehyde exposure. Estimates of the relative risk of experiencing formaldehyde-related symptoms in the anatomy laboratories compared to the control laboratories ranged from 2.0-19.0, depending on the particular symptom. In addn, it was found that female students were 3 times more likely to report formaldehyde-related symptoms than male students.
[Fleischer JM; NY J Med 87 (7): 385-8 (1987)]**PEER REVIEWED**
A population based case control study was undertaken in 13 counties of western Washington to determine if occupational formaldehyde exposure was related to cancer of the oropharynx & hypopharynx (OHPC, N=205), nasopharynx (NPC, N=27) or sinus & nasal cavity (SNC, N=53). Controls were selected by random digit dialing (N= 552). A telephone interview inquired about lifetime occupational history as well as a number of potential confounding factors, including smoking & drinking. Approximately half (N=143) of the case interviews were with next of kin. ... Logistic regression was used to estimate exposure odds ratios STET while taking into account multiple risk factors for each site. No significant associations were found between occupational formaldehyde exposure & any of the cancer sites under study. However, relative risk estimates associated with the highest exposure score categories were evaluated for oropharynx & hypopharynx (OR=1.3, 95% Confidence Interval= 0.6-3.1) & nasopharynx (OR=2.1, 95% Cl=0.4-10.0). When an induction period was accounted for only oropharynx & hypopharynx & nasopharynx increased to 1.7 & 3.1, respectively. Several limitations in the study tend to conservatively bias the results. ...
[Vaughn TL et al; Int J Cancer 38 (5): 677-84 (1986)]**PEER REVIEWED**
Because of the paucity of scientific data concerning the inhalation toxicity of formaldehyde in humans, determinations of the symptoms & alterations in pulmonary function resulting from inhalation for 1 hr of 3 ppm formaldehyde were studied. The protocol consisted of randomized exposure of each subject to clean air or 3.0 ppm formaldehyde on 2 separate days. Twenty-two healthy normal subjects engaged in intermittent heavy exercise (VE= 65/min) & 16 asthmatic subjects performed intermittent moderate exercise (VE= 37/min). Symptoms & pulmonary functions were assessed during the time course of exposure; nonspecific airway reactivity was assessed after exposure. Both groups exhibited similar, significant (p<0.01) increases in perceived odor, nose/throat irritation, & eye irritation throughout the exposure. The non-asthmatic group had the following slight but statistically significant (p<0.02) lower pulmonary functions after 55 min of exposure to formaldehyde as compared to clean air: 3.8% in FEV1, 2.6% in FVC, & 2.8% in FEV3. The asthmatic group showed no statistically significant decrements in pulmonary function.
[Green DJ et al; Am Rev Respir Dis 135 (6): 1261-6 (1987)]**PEER REVIEWED**
A retrospective mortality analysis was conducted in a cohort of 9,365 individuals employed as of 1940 in two chrome leather tanneries in the United States and followed to the end of 1982. Vital status as of the closing date was determined for over 95% of the cohort. Potential hazardous workplace exposures varied with department and included ... formaldehyde. ... Mortality from all causes combined was lower than expected for each tannery. ... Deaths from cancer of each site, including the lung, were also lower than expected compared to those of either the population of the United States or of local state rates. A significant excess of deaths was observed, however, due to accidental causes in one tannery and cirrhosis of the liver, suicide, and alcoholism in the other. These excesses did not appear to be casually associated with occupational exposures.
[Stern FB et al; Scand J Work Environ Health 13 (2): 108-17 (1987)]**PEER REVIEWED**
Infectivity of human T-cell lymphotropic virus, Type III (HTLV-III) was ... efficiently inactivated by formalin ... .
[Quinnan GV et al; Transfusion 26 (5): 481-3 (1986)]**PEER REVIEWED**
Eight symptomatic individuals chronically exposed to indoor formaldehyde at low concentrations (0.07-0.55 ppm) were compared to 8 nonexposed subjects with respect to: (1) presence of IgG and IgE antibodies to formaldehyde conjugated to human serum albumin (F-HSA); (2) the percentage of venous blood T- and B-cells by E- and EAC-rosetting; and (3) the ability of T- and B-cells to undergo mitogen (phytohemagglutin and pokeweed) stimulated blastogenesis as measured by the incorporation of tritiated thymidine. Anti-F-HSA IgG, but not IgE, antibodies were detected in the sera of the 8 exposed subjects; none were found in 7 of the controls. T-lymphocytes were decreased in the exposed (48%) compared to the control (65.9%) subjects (p< 0.01). B-cells were 12.6% (exposed group) and 14.75% (controls) (p< 0.05). The incorporation of labeled thymidine by T-cells (phytohemagglutin) was decreased: 17,882 cpm (exposed group) and 28,576 cpm (p< 0.01). T- and B-cell blastogenesis (pokeweed) was 9,698 cpm (exposed group) and 11,279 (controls) (p< 0.1).
[Thrasher JD et al; Arch Environ Health 42 (6): 347-50 (1987)]**PEER REVIEWED**
Both death and survival from 4-oz formalin ingestions have been reported in adults. The probable mean lethal adult dose is 1 to 2 oz. Death may occur within 3 hours; survival past 48 hours usually means recovery.
[Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1214]**PEER REVIEWED**
An environmental survey of 2 wood products (plywood, particle-board) companies revealed mean concns in the plywood forming areas of 0.8 ppm &, in 2 particle-board forming areas, of 1.1 to 1.4 ppm /formaldehyde/. Ophthalmologic evaluations were conducted & eye irritation self-reports were collected from 84 subject workers, including unexposed controls, from various areas in the plants. Results from both were unremarkable, as were tests mapping their visual fields. However, there were subjective reports of at least occasional eye irritation in 67% of the exposed subjects, with more such reports coming from workers in areas of the plant with the higher concns. An explosion at the factory closed a major product line & resulted in laying off many of the volunteer subjects prior to performance testing; the remaining 49 workers were tested before & after their workshift (& 13 of them were tested on 2 days) in order to assess acute effects of formaldehyde on visual acuity, depth perception, peripheral vision, accommodation, eye movement & fixation, divided attention, & color vision. Subjective reports of eye irritation on the day of testing did not correlate, or correlated negatively, with formaldehyde concns on the test day, which averaged 0.4 ppm. Average visual test scores were better at the end of the day than at the beginning, & there was a trend for those with higher formaldehyde levels to demonstrate greater improvement. Some of the changes reached traditional levels of statistical significance. The results from this investigation, while relevant to the neurotoxicity of formaldehyde, suffer from the small sample size & the possibility that the comparison subjects had also experienced formaldehyde exposure. With these caveats, this study suggests that mean formaldehyde exposures at 0.4 ppm produce no deleterious acute effects on visual performance, but chronic exposures between 0.8 & 1.4 ppm may produce an increased incidence of self reported symptoms of eye irritation in persons who do not have clinical ophthalmologic defects.
[O'Donoghue, J.L. (ed.). Neurotoxicity of Industrial and Commercial Chemicals. Volume I. Boca Raton, FL: CRC Press, Inc., 1985., p. 59]**PEER REVIEWED**
Symptoms: Local: Conjunctivitis, corneal burns; brownish discoloration of skin; dermatitis, urticaria (hives), pustulovesicular eruption. Inhalation: rhinitis & anosmia (loss of sense of smell); pharyngitis, laryngospasm; tracheitis & bronchitis; pulmonary edema, cough, constriction in chest; dypsnea (difficult breathing), headache, weakness, palpitation (rapid heart beat), gastro enteritis (inflammation of the stomach & intestines). Ingestion: Burning in mouth & esophagus; nausea & vomiting; abdominal pain, diarrhea, vertigo (dizziness), unconsciousness, jaundice, albuminuria, hematuria, anuria, acidosis, convulsions.
[ITII. Toxic and Hazardous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute, 1988., p. 249]**PEER REVIEWED**
Aldehydes increase airflow at concentrations below those that decrease respiratory frequency. /Aldehydes/
[Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1618]**PEER REVIEWED**
Data on concentration of formaldehyde and 15 organic solvents in Finnish furniture factories from 1975 to 1984 were presented. Workers often complained of severe eye, nose, and upper respiratory tract irritation. Formaldehyde was collected in a 1% sodium bisulfite solution and analyzed by the chromatropic method. The solvents were adsorbed in a charcoal tube, desorbed with carbon-disulfide or dimethylformamide, and analyzed by gas chromatography. All highly exposed workers were monitored. The widest range of formaldehyde concentration was recorded in the operation of the curtain painting furniture receiving operation, which was between 0.2 and 5.4 ppm. The mean concentrations of most organic solvents studied ranged from 4 to 66 ppm. Formaldehyde levels were high and the 1 ppm exposure limit, defined as the 15 minute time weighted average by the Finnish Board of Labor Protection, was exceeded about 40% of the time.
[Priha E et al; Ann Occup Hyg 30 (3): 289-94 (1986)]**PEER REVIEWED**
A study of 759 histologically verified cancers of the nasal cavity (287 cases), paranasal sinuses (179 cases), and nasopharynx (293 cases) and 2465 cancer controls diagnosed in Denmark between 1970 and 1982 was conducted to investigate the importance of occupational exposure to formaldehyde. Information on job history for cases and controls was derived from a national data linkage system and exposure to formaldehyde and wood dust was assessed by industrial hygienists unaware of the case control status of the patients. The exposure rates for formaldehyde among male and female controls were 4.2% and 0.1% respectively. After proper adjustment for contemporary wood dust exposure, relative risk of 2.3 (95% CI= 0.9-5.8) for squamous cell carcinoma and 2.2 (95% CI= 7-7.2) for adenocarcinoma of the nasal cavity and paranasal sinuses were detected among men who have been exposed to formaldehyde in their job compared with those never exposed.
[Olsen JH, Asnaes S; Br J Ind Med 43 (11): 769-74 (1986)]**PEER REVIEWED**
The National Cancer Institute study on the relationship between exposure to formaldehyde & mortality from nasophryngeal cancer was evaluated. The study had indicated little evidence of a link between formaldehyde at concns normally encountered in the workplace & risk of nasopharyngeal cancer. Although the overall standardized mortality ration was significantly elevated in subjects exposed to formaldehyde, the overall risk did not incr with increasing intensity of exposure. A reanalysis, however, suggested that simultaneous exposure to particulates & formaldehyde could be a risk factor. A further review of the National Cancer Institute findings showed that the significant excess mortality was based on deaths occurring in a single factory (factory-A) & occurred primarily in short term employees. When the data were analyzed in terms of cumulative exposures that were known to include both formaldehyde & particulates, only the highest exposure group had a significantly increased excess nasopharyngeal cancer mortality. This excess was clearly located in factory-A. A follow-up study of factory-A that added 5 more years of follow-up was initiated. It showed no additional deaths from nasopharyngeal cancer even among workers with the highest formaldehyde & particulate exposures. The four deaths from nasopharyngeal cancer in this factory occurred in workers employed in the same department & hired between 1949 & 1955. Although these workers were exposed to formaldehyde & particulates, they were not among the most highly exposed.
[Collins JJ et al; J NCI 80 (5): 376-7 (1988)]**PEER REVIEWED**
This study evaluates the histological changes, especially the presence of possible precancerous lesions, in the nasal mucosa of workers exposed to formaldehyde. Nasal biopsies of 37 workers occupationally exposed to formaldehyde for more than five years and 37 age matched referents showed a higher degree of metaplastic alterations in the former group. In addition, three cases of epithelial dysplasia were observed among the exposed. These results indicate that formaldehyde may be potentially carcinogenic in man. Combination of this finding with the inconclusive epidemiological studies suggests that formaldehyde is a weak carcinogen and that occupational exposure to formaldehyde alone is insufficient to induce nasal cancer.
[Boysen M et al; Br J Ind Med 47 (2): 116-21 (1990)]**PEER REVIEWED**
Clinical & animal studies suggest that formaldehyde adsorbed on respirable particles may elicit a greater pulmonary physiologic & inflammatory effect than gaseous formaldehyde alone. This study was to determine if respirable carbon particles have a synergistic effect on the acute symptomatic & pulmonary physiologic response to formaldehyde inhalation. Normal, nonsmoking, methacholine-nonreactive subjects were exposed to 2 hr each of clean air, 3 ppm formaldehyde, 0.5 mg/cu m respirable activated carbon aerosol, & the combination of 3 ppm formaldehyde plus activated carbon aerosol. The subjects engaged in intermittent heavy exercise (VE= 57 1/min) for 15 min each half hour. Formaldehyde exposure was associated with significant increases in reported eye irritation, nasal irritation, throat irritation, headache, chest discomfort, & odor. Synergistic increases in cough, but not in other irritant respiratory tract symptoms, were observed with inhalation of formaldehyde & carbon. Small (<5%) synergistic decreases in FVC & FEV3 were also seen. No formaldehyde effect was observed on FEV1; however, we did observe small (<10%) significant decreases in FEF25-75%, which may be indicative of increased airway tone. Overall, results demonstrated synergism, but the effect is small & its clinical significance is uncertain.
[Green DJ et al; J Toxicol Environ Health 28 (3): 261-75 (1989)]**PEER REVIEWED**
To study the cytotoxic effect of formaldehyde on the human nasal mucosa 75 men with occupational exposure to formaldehyde or to formaldehyde & wood dust, were examined, looking particularly at early signs of irritative effects & histopathological changes in the nasal mucosa. A nasal biopsy specimen was graded from 0-8 according to the morphological changes. A high frequency of nasal symptoms, mostly a running nose & crusting, was related to exposure to formaldehyde. Only three men had a normal mucosa; the remainder has loss of cilia & goblet cell hyperplasia (11%) & squamous metapolasia (78%); in 6 cases (8%) there was a mild dysplasia. The histological grading showed a significantly higher score when compared with unexposed controls (2.9 v 1.8). There was no dose response relation, no malignancies, & no difference in the histological score between those exposed to formaldehyde or to formaldehyde & wood dust.
[Edling C et al; Br J Ind Med 45 (11): 761-5 (1988)]**PEER REVIEWED**
A study of respiratory symptoms and pathophysiological effects associated with occupational exposure to formaldehyde and wood dust was conducted. The cohort consisted of 70 Swedish workers exposed to formaldehyde during the production of formaldehyde and formaldehyde based products (formaldehyde group) and 100 furniture workers exposed to formaldehyde and wood dust (formaldehyde/wood dust group). The comparisons consisted of 36 local government clerks. The formaldehyde group was exposed to 0.05 to 0.5 mg/cu m formaldehyde and the furniture workers to 0.2 to 0.3 mg/cu m formaldehyde and 1 to 2 mg/cu m wood dust. Annual formaldehyde exposures of the comparisons averaged 0.09 mg/cu m. Sixty four percent of the formaldehyde group, 53% of the formaldehyde/wood dust group, and 25% of the comparisons reported nasal discomfort. Symptoms from the lower airways were reported by 44% of the formaldehyde group, 39% of the formaldehyde/wood dust group, and 14 % of the comparisons. Symptoms of nasal obstruction and watery discharges were more frequent in the exposed subjects than in the comparisons. More pronounced nasal swelling was found in the cohort than in the comparisons. 20% of the formaldehyde and 15% of the formaldehyde/wood dust group had impaired mucociliary clearance versus only 3% of the comparisons. Both exposed groups had a reduced sense of smell. Forced vital capacity was significantly decreased in the exposed groups.
[Holmstorm M, Wilhelmsson B; Scandinavian J Work Environ Health 14 (5): 306-11 (1988)]**PEER REVIEWED**
A study was conducted to determine if pathologists with exposure to formaldehyde demonstrate an excess risk of cancer, particularly cancers of the nasopharyngeal and pharyngeal areas. A population of 6411 physicians with occupational formaldehyde exposure participated in the study. The occurrence of these types of cancers was 4.7 times higher in these persons than in a comparable sized group of psychiatrists, but even so it is difficult to determine the importance of this increased risk as being directly tied to formaldehyde exposure. Pathologists and other members of the study group were exposed to other chemicals and infectious agents as well as formaldehyde. There was an apparent excess of mortality from pancreatic cancer and brain cancers as well as leukemia.
[Matanoski GM; Risks of Pathologists Exposed to Formaldehyde School of Hygiene and Public Health, Department of Epidemiology, Johns Hopkins University, Baltimore, Maryland, Grant No. RO1-OH-01511 (1989)]**PEER REVIEWED**
The relation of chronic respiratory symptoms & pulmonary function to formaldehyde in homes was studied in a sample of 298 children (6-15 yr of age) & 613 adults. Formaldehyde measurements were made with passive samplers during two 1 wk periods. Significantly greater prevalence rates of asthma & chronic bronchitis were found in children from houses with formaldehyde levels 60-120 ppb than in those less exposed, especially in children also exposed to environmental tobacco smoke. In children, levels of peak expiratory flow rates decreased linearly with formaldehyde exposure, with the estimated decr due to 60 ppb of formaldehyde equivalent to 22% of peak expiratory flow rates level in nonexposed children. The effects in asthmatic children exposed to formaldehyde below 50 ppb were greater than in healthy ones. The effects in adults were less evident: decrements in peak expiratory flow rates due to formaldehyde over 40 ppb were seen only in the morning, & mainly in smokers.
[Krzyzanowski M et al; Environ Res 52 (2): 117-25 (1990)]**PEER REVIEWED**
The long term effects of formaldehyde on the respiratory tract have been investigated in a group of 164 workers exposed daily to the chemical during the production of urea formaldehyde resin, together with 129 workers not exposed to free formaldehyde. Exposure was classified as high (corresponding to an 8 hr time weighted exposure of >2.0 ppm), medium (0.6-2.0 ppm), or low (0.1-0.5 ppm). 25% of workers had high exposure at some time & 17% moderate exposure. Both exposed & unexposed groups had an annual assessment that included lung function. The proportion with self reported respiratory symptoms was similar in the two groups, 12% & 16% reporting breathlessness on hurrying & 26% & 20% wheezing. The initial forced expiratory volume in 1 sec was within 0.5 l (approx on standard deviation) of the predicted value (by age & height) in 65% of the exposed & 59% of unexposed workers & >0.5 l below the predicted value in 9% of exposed & 11% unexposed workers. The mean decline in forced expiratory volume in 1 sec was 42 ml/yr (standard deviation 45) in the exposed & 41 ml/yr in the unexposed group (standard deviation 40 ml/yr). The rate of decline showed the expected association with smoking in the unexposed group, but in the exposed group the mean rate of decline in the never smokers was similar to that in current smokers. There were, however, relatively few never smokers & considerable variation in the rates of decline. In the exposed group no association was found between the rate of decline & indices of exposure to formaldehyde. Thus there is no evidence from this study of an excess of respiratory symptoms or decline in lung function in the workers exposed to formaldehyde. The similar rate of decline of forced expiratory volume in 1 sec however in never smokers & smokers of the exposed group is consistent with finding of other studies for workers exposed to formaldehyde.
[Nunn AJ et al; Br J Ind Med 47 (11): 747-52 (1990)]**PEER REVIEWED**
A prospective evaluation of pulmonary function & respiratory symptoms was conducted among 103 medical students exposed to formaldehyde over a 7 month period to determine the incidence of bronchoconstriction & respiratory symptoms in response to exposure. Time-weighted average formaldehyde exposures were generally <1 ppm & peak exposures were <5 ppm. Acute symptoms of eye & upper respiratory irritation were significantly associated with exposure. There was no pattern of bronchoconstriction in response to exposure after either 2 wks or 7 months. Twelve subjects had a history of asthma; they were likely to have symptoms of respiratory irritation or changes in pulmonary function than those without such a history. These findings are consistent with previous case reports that indicate exposure to formaldehyde vapor at levels that are commonly encountered in occupational & residential seetings do not commonly cause significant bronchonconstriction, even among subjects with preexisting asthma.
[Uba G et al; Am J Ind Med 15 (1): 91-101 (1989)]**PEER REVIEWED**
A case of anaphylactoid reaction to a patch test with formaldehyde was described. The 40 year old woman developed bronchospasm and laryngospasm following the inhalation of formaldehyde vapor. A year later she accidentally entered a hospital room relatively soon after it had been disinfected, and was hospitalized with dyspnea, cyanosis, bronchospasm, and laryngospasm. Days later she did react to a patch test with a 1% solution of formaldehyde in water. Pulmonary function tests 20 min after the patch test revealed a 50% reduction in FEV1 and a 63% reduction in MEF 25.
[Orlandini A et al; Contact Dermatitis 19 (5): 383-4 (1988)]**PEER REVIEWED**
Four groups of patients with long-term inhalation exposure to formaldehyde were compared with controls who had short-term periodic exposure to formaldehyde. The following were determined for all groups: total white cell, lymphocyte, and T cell counts; T helper/suppressor ratios; total Ta1+, IL2+, and B cell counts; antibodies to formaldehyde-human serum albumin conjugate and autoantibodies. When compared with the controls, the patients had significantly higer antibody titers to formaldehyde-human serum albumin. In addition, significant increases in Ta1+, IL2+, and B cells and autoantibodies were observed. Immune activation, autoantibodies, and anti formaldehyde-human serum albumin antibodies are associated with long-term formaldehyde inhalation.
[Thrasher JD et al; Arch Environ Health 45 (4): 217-23 (1990)]**PEER REVIEWED**
The incidence of spontaneous abortions among hospital staff who used ethylene oxide, glutaral (glutaraldehyde) & formaldehyde for the chemical sterilization of instruments was studied using data from a questionnaire & a hospital discharge register. ... When the staff were concerned in sterilizing during their pregnancy the frequency was 16.7% compared with 5.6% for the nonexposed pregnancies. The incr frequency ... correlated with exposure to ethylene oxide but not with exposure to glutaral or formaldehyde.
[Hemminki K et al; Brit Med J 285: 1461-63 (1982)]**PEER REVIEWED**
Employees exposed to formaldehyde in the woodworking industry and nonexposed control subjects were examined by spirometry and the nitrogen washout technique. A dose-response relationship was found between exposure to formaldehyde and decrease in lung function. Industrial exposure to formaldehyde causes transient lung function impairment over a work shift, with a cumulative effect over the years. The impairment, however, can be reversed with 4 wk of no exposure.
[Alexandersson R, Hedenstierna G; Arch Environ Health 44 (1): 5-11 (1989)]**PEER REVIEWED**
The mortality of 1,332 male workers employed at least 30 days in 1959-1980 in a resins-manufacturing plant was examined. Ambient measurements taken in the plant between 1974 and 1979 documented a potential for exposure to levels of formaldehyde as high or greater than 3.0 mg/cu m. Vital status was ascertained for 98.6% of the cohort members, and their mortality was compared with expected deaths drawn from the national and local population rates. A statistically significant increase in lung cancer was observed, based on 18 deaths, which was not fully accounted for by possible confounding factors linked to personal habits or sociocultural characteristics. This elevated risk, however, could not be attributed specifically to exposure to formaldehyde. Mortality from digestive cancer (14 deaths observed) and hematologic neoplasms (5 deaths observed) was not substantially higher than expected.
[Bertazzi PA et al; Scand J Work Environ Health 12 (5): 461-8 (1986)]**PEER REVIEWED**
Formaldehyde has been found to cause bronchial asthma-like symptoms in humans. A young male neurology resident who spent 2 hr in autopsy of formaldehyde-preserved human brains experienced both conjunctival & nasal irritation while working; however, over the next 15 hr after cessation of exposure, he developed progressive dyspnea & tightness in the chest. Early edema indicative of pneumonitis was visible on Xray, & after treatment with aminophyline, hydrocortisone, & oxygen (nasal prong at 4 l/min), he gradually improved over the following 2 days. He continued to need prednisone (20 mg every other day for 2 wk), & he had fully recovered 5 wk after the onset of his hypersensitivity reaction to inhaled formaldehyde.
[American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991., p. 677]**PEER REVIEWED**
In cultured human bronchial fibroblasts exposed to the carcinogen N-methyl-N-nitrosourea (NMU) in combination with formaldehyde, formaldehyde was observed to inhibit repair of alkylation of DNA at the O6 guanine position induced by NMU. Whether formaldehyde enhances the effects of other DNA-damaging agents has not yet been evaluated.
[Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 868]**PEER REVIEWED**
Hemodialysis patients are exposed chronically to trace levels of formaldehyde (by formalin sterilization of their dialyzers to permit reuse). Erythrocytes can be characterized in terms of MN phenotypes, analogous to the AB-O system. The normal distribution of MM, NN, an MN phenotypes is about 25, 25, and 50%, respectively. Only 25% of the population would be expected to have anti-N antibodies. Formaldehyde exposure may be followed by the development of anti-N-like antibodies probably as a result of reaction with the dissolved form of formaldehyde, methylene glycol. The anti-N-like antibodies are also found following exposure to sodium hypochlorite.
[Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1216]**PEER REVIEWED**
The use of formaldehyde as a nail hardener, on the other hand, is accompanied by a significant number of serious injuries to sensitive nail and adnexal tissues. This type of exposure may contribute substantially to that portion of the 4% sensitization index seen in clinical patients which is cosmetic-related.
[Marzulli, F.N., H.I. Maibach. Dermatotoxicology 4th ed. New York, NY: Hemisphere Publishing Corp., 1991., p. 424]**PEER REVIEWED**
In a study ..., a group of 33 observers judged the perceived irritation & odor of formaldehyde during 29-min chamber esposures to concns ranging from 0.3-2.4 mg/cu m. The sensory irritation increased with time for the lower concns & decreased with time for the highest. This effect was true for irritation of eyes, nose, & throat & the sensitivity proved to be roughly equal for all three sites. The sensory irritant effect of formaldehyde at 1.2 mg/cu m was shown to decr when the chemical pyridine was injected into the chanber; such sensory interactions occur in environmentally realistic situations.
[WHO; Environ Health Criteria 89: Formaldehyde p.138 (1989)]**PEER REVIEWED**
... Healthy volunteers (24 men, 9 women) /were exposed/ to formaldehyde concns ranging between 0.036 & 4.8 mg/cu m air (33 volunteers for 35 min, 48 volunteers for 1.5 min. Eye blinking rates as well as subjective irritation effects were determined. The irritation threshold was found to range between 1.2 & 2.4 mg formaldehyde/cu m. A similar threshold (1 mg/cu m) was found in other studies. ... /It was/ noted that 9 out of 53 medical student volunteers exposed to formaldehyde concns of between 0.39 & 0.60 mg/cu m for 8 hr/wk, complained of headaches, a burning sensation in the eyes, sore throat, & annoyance because of the smell.
[WHO; Environ Health Criteria 89: Formaldehyde p.138 (1989)]**PEER REVIEWED**
A 60-yr old man swallowed 60-90 mg of a 40% formaldehyde soln. Thirty hr after death, the mucosa of the lowere part of the esophagus, stomach, & first portion of duodenum were dark chocolate brown in color & of the consistency of leather. All organs & tissues in contact with the stomach were "hardened" to a depth of about 8 mm.
[WHO; Environ Health Criteria 89: Formaldehyde p.141 (1989)]**PEER REVIEWED**
Workers exposed to 0.35-1.0 ppm (0.43-1.2 mg/cu m) for 6 minutes had a significant irritation response at 1.0 ppm; nonsignificant responses were reported at 0.7 and 0.9 ppm(0.9 and 1.1 mg/cu m).
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V62 303 (1995)]**PEER REVIEWED**
Formaldehyde vapor is very irritating to the mucous membranes and toxic to animals, including man.
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 525]**PEER REVIEWED**
... examined smears of nasal respiratory mucosa cells sampled from the inner turbinate of 15 nonsmokers who were exposed to formaldehyde released from a urea-formaldehyde glue used in a plywood factory and 15 age- and sex-matched nonexposed clerks from outside of the factory. Estimates of formaldehyde air conc ranged from : 0.21 to 0.60 (mean 0.39 + or - 0.20 ppm) in the warehouse where seven subject worked, 0.08 to 0.14 ppm (mean 0.1 + or - 0.02 ppm) in the shearing press where six subjects worked, and 0.09 ppm (only one sample taken) in the sawmill area where two subjects worked. Mean wood dust concn for the three areas were 0.23 + or - 0.1 mg/m3, 0.41 + or - 0.21 mg/m3, and 0.73 mg/m3, respectively. Exposed subjects worked at the factory for 2-19 yr (mean 6.8 + or - 5.0 yr). Nasal mucosal slides were scored as follows: normal cellularity, 1; number of mucus-secreting cells greater than ciliated cells, 1.5; hyperplasia, 2; squamous metaplasia, 2.5; mild dysplasia, 3; moderate dysplasia, 4; severe dysplasia, 5; and malignant cells, 6. In the exposed group, all subjects had a greater number of nonciliated than ciliated cells, 40% had hyperplasia, 67% had squamous metaplasia, and 6% slight dysplasia. In controls, 26% had normal cytology, 67% had more ciliated than nonciliated cells, 33% had hyperplasia, and 6% had squamous metaplasia. The mean cytology score for the exposed group (2.3 + or - 0.5) was reported to be statistically significantly greater than the control score (1.6 + or - 0.5). Also found in this study was a statistically significantly higher percentage of micronucleated mucosal cells in the exposed group compared with the control group (0.91% + or - 0.47 versus 0.25% + or - 0.22).
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 51 (1999)]**PEER REVIEWED**
Mean baseline PEFR /(peak expiratory flow rate)/ declined by about 2% over a 10-wk period in a group of 24 physical therapy students who dissected cadavers for 3-hr periods/wk ... . Estimates of breathing zone formaldehyde concn ranged from 0.49-0.93 ppm (geometric mean 0.72 + or - 1.22 ppm). PEFR, the only pulmonary function variable measured in this study, was measured before & after each exposure period. Postexposure PEFR means were 1-3% lower than preexposure PEFR means during the first 4 wk, but this difference was not apparent during the last 6 wk. Fourteen wk after the end of the 10-wk period, the mean PEFR for the group returned to the preexposure baseline value.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 53 (1999)]**PEER REVIEWED**
... evaluated the immunologic response of asthmatic subjects exposed to urea-formaldehyde foam insulation (UFFI) off-gas products. Subjects consisted of 23 individuals with a history of asthmatic symptoms attributed to UFFI & 4 individuals (controls) with asthma unrelated to UFFI by-products. Subjects were exposed to one of the following: room air (placebo) for 30 min; 1 ppm formaldehyde gas for 3 hr; UFFI particles (4 um, 0.5 particles/ml) for 3 hr, commencing 48 hr after formaldehyde gas exposure; & UFFI off-gas products for 3 hr, commencing 48 hr after UFFI particle exposure. There were no significant alterations in any of the white blood cell populations ... . However, there was a significant incr in the % & absolute number of eosinophils & basophils in the subject (who also lived in UFFI-homes) after exposure to UFFI in the exposure chamber when compared to the white blood cell values obtained before chamber exposure to UFFI.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 66 (1999)]**PEER REVIEWED**
Occupational exposures to formaldehyde have been assoc with dermal irritation and the diagnosis of allergic contact dermatitis by patch testing. Reported historical percentages of subjects with skin problems showing positive responses to formaldehyde in patch tests performed by dermatologists using aqueous soln with 1 or 2% formaldehyde incl 7.8% in North America between 1992 and 1994 ... 1.6% in a 1983-1984 Swedish study ... 2.6% in a 1988-1989 European study ... and 3.7% in a 1990-1994 Polish study ... . Lack of case-specific exposure info for these patients precludes the determination of the degree to which sensitization may have been caused by direct dermal contact to formaldehyde in liquids or by contact with formaldehyde gas in air, but the widespread use of formaldehyde or formaldehyde-releasing chemicals in cosmetics and cleaning agents ... suggest that the dermal route of exposure may be the more important sensitizing route.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 69 (1999)]**PEER REVIEWED**
... measured elevated levels of formaldehyde-specific IgE in 24/62 8-yr old children who were students in three particle board-paneled classrooms with est formaldehyde air concn of 0.075, 0.069, and 0.043 ppm. In a health survey, the children reported headaches (29/62), fatigue (21/62), dry nasal mucosa (9/62), rhinitis (23/62) cough (15/62), and nosebleeds (14/62). Sums of numbers of children with each of nine symptoms for each classroom decr with decr formaldehyde conc (49, 47, and 24, respectively for the 0.075-, 0.069-, and 0.043-ppm classrooms), but the investigators reported that elevated levels of specific IgE did not correlate with the number and severity of symptoms. The children were moved to a new school without particle board paneling and were evaluated again, 3 mo after moving. Est formaldehyde concn in the new classrooms were 0.029, 0.023, and 0.026 ppm. The numbers of children reporting symptoms decr significantly compared with premoving reporting figures, and mean serum levels of formaldehyde-specific IgE, measured in 20 of the children, declined significantly compared with premoving mean levels.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 74 (1999)]**PEER REVIEWED**
... investigated the correlation between formaldehyde-induced contact dermatitis and granulocyte chemiluminescence resulting from free-radical release in healthy and formaldehyde-sensitive patients. Thirteen patients with contact dermatitis who were occupationally exposed to formaldehyde and five healthy volunteers participated in the study. All subjects underwent skin-prick tests for common allergens as well as a histamine inhalation provocation test. Subjects were exposed to 0.5 mg/m3 (0.41 ppm) formaldehyde for 2 hr, and peak expiratory flow was measured immediately before exposure, at 60 and 120 min of exposure, and at 6 and 21 hr after completion of exposure. In formaldehyde-sensitive patients, skin-prick tests and total serum IgE were normal; no antiformaldehyde IgE was detected. In formaldehyde-sensitive patients, peripheral blood granulocyte chemiluminescence significantly incr within 30 min of exposure commencement, and remained elevated 24 hr later, compared to initial values. Granulocyte chemiluminescence did not incr in healthy patients.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 75 (1999)]**PEER REVIEWED**
... measured the formation of DNA-protein cross links in peripheral white blood cells of occupationally exposed workers (n=12) & unexposed controls (n=8). The avg length of ... exposure was 13 yr. ... Venous blood samples were collected ... . Personal & room concn of formaldehyde were collected at various periods during the working day among the exposed subjects, with formaldehyde room concn ranging from 1.38-1.6 ppm. Personal monitoring devices indicated formaldehyde concn of 2.8-3.1 ppm during peak work & an avg concn of 1.46 ppm at times when work was usually completed. Exposure to formaldehyde resulted in a significant incr in the incidence of DNA-protein cross links. Mean ... incidences in exposed & nonexposed workers were 28 + or - 6 & 22 + or - 6%, respectively. Within the exposed workers group, technicians had significantly greater levels of DNA-protein cross links than physicians (32.3 + or - 4.3 & 26.3 + or - 4.4%, respectively). A linear relationship between yr of exposure & DNA-protein cross links formation was also detected. When the data were analyzed considering worker smoking habits, DNA-protein cross links was consistently elevated among formaldehyde-exposed versus corresponding controls (p=0.03).
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 86 (1999)]**PEER REVIEWED**
The finding of nasal tumors in rodents exposed to high levels of airborne formaldehyde in the early 1980s ... led to a concern for cancer effect in occupationally exposed workers. There are now more than 40 epidemiology studies examining the potential for occupational formaldehyde exposure to cause cancer in humans. The studies include cohort mortality studies of formaldehyde-exposed industrial workers, cohort mortality studies of formaldehyde-exposed professionals or medical specialists, & case-control studies that looked for assoc between occupational exposure to formaldehyde & cancers of the nose, pharynx, or lung. ... Although some of the epidemiological studies have found some scattered evidence for extra-respiratory site cancers in groups of formaldehyde-exposed workers, the data are not consistent across studies & adjustment for potential confounding cancer risk factors has not often been possible. Most, if not all reviewers, have agreed that cancer of the respiratory tract, particularly the upper respiratory tract, is more biologically plausible than formaldehyde-induced cancer at distant sites given the reactivity of formaldehyde, the capacity of tissues to metabolize formaldehyde, & the results from chronic rodent inhalation studies showing that formaldehyde-induced nonneoplastic & neoplastic effects are restricted to the upper respiratory tract with exposures to concn below 5-10 ppm. Accordingly, the meta-analyses of the human data have focused on the findings for respiratory cancer deaths in occupationally exposed humans.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 89 (1999)]**PEER REVIEWED**
... describe the case of a 58-yr old man who swallowed 4 ounces of formalin (517 mg formaldehyde/kg) in a suicide attempt. The man was found unconscious by a co-worker about 1 hr after his shift began. In the emergency room, the subject regained consciousness but was lethargic. Lab results indicated significant acidosis. Approx 3 hr after ingesting the formalin, the patient complained of abdominal pain & began retching without emesis; he was admitted for observation & treated with ethanol. The patient's abdominal pains became more severe & he had difficulty breathing. At 5.5 hr after ingestion, the patient became obtund, & both his respiratory rate & blood pressure fell significantly; he was intubated & placed on 100% oxygen. Shortly thereafter, the patient began to experience seizures; treatment with diazepam & phenytoin was unproductive, but pancuronium was effective in treating the seizures. IV bicarbonate & ethanol therapies were begun after the seizures started. The patient was transported for dialysis, but on arrival, had clinical signs of intravascular coagulopathy. He subsequently sustained a cardiac arrest from which he could not be revived. At autopsy, the patient's stomach was hard, white, & leathery; the esophagus & intestines appeared to be normal.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 113 (1999)]**PEER REVIEWED**
A 55-yr old woman and a 34-yr old man ingested, with suicidal intent, an unknown amt of what was reported to have been formalin ... . The female patient was found in a coma and admitted to the hospital with shock (systolic blood pressure 50 mm Hg), respiratory insufficiency, and metabolic acidosis. The male patient, who had a history of alcohol abuse, was also hospitalized with shock (systolic blood pressure 60 mm Hg), respiratory insufficiency, and metabolic acidosis. Both patients underwent hemodialysis and hemofiltration treatment. Analysis of the formaldehyde samples ingested by both patients showed no evidence that these products contained methanol, although it was expected to have been detected. A chemical-toxicological screening /of blood samples/ indicated that no drugs other than formaldehyde had been ingested ... . Three wk after ingestion of formaldehyde, the female patient died of cardiac failure refractory to catecholamine therapy. The male patient developed adult respiratory distress syndrome and died 8 wk after formaldehyde ingestion with signs of cardiac failure.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 113 (1999)]**PEER REVIEWED**
Human lymphoblast mutants at the X-linked hprt locus have been examined by Southern blot, Northern blot & DNA sequence analysis. A previous study had shown that approx a third of the spontaneously arising mutants & half those induced by formaldehyde showed no alteration in restriction fragment pattern & thus were classified as point mutation. In this report, these point mutants fall into 4 catagories: normal size & amount of RNA, normal size but reduced amounts, reduced size RNA or no RNA. Sequence analyses of cDNAs prepared from hprt mRNAs were performed on 1 spontaneous & 7 formaldehyde induced mutants were base substitutions, all of which occurred at AT base-pairs. There was an apparent hot spot, in that 4/6 independent mutants were AT----CG transversions at one specific site. The remaining mutant had lost exon 8.
[Liber HL et al; Mutat Res 226 (1): 31-7 (1989)]**PEER REVIEWED**
Human Toxicity Values:
The probable mean lethal adult dose is 1-2 oz.
[Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1214]**PEER REVIEWED**
Skin, Eye and Respiratory Irritations:
Contact with the skin causes irritation, tanning effect, and allergic sensitization. Contact with eyes causes irritation, itching, & lacrimation. ...
[Environment Canada; Tech Info for Problem Spills: Formaldehyde p.2 (1985)]**PEER REVIEWED**
Formaldehyde vapor is very irritating to the mucous membranes and toxic to animals, including man.
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 525]**PEER REVIEWED**
Medical Surveillance:
Consider the skin, eyes, & resp tract in any placement or periodic examination, esp if the patient has a history of allergies.
[Sittig, M. Handbook of Toxic and Hazardous Chemicals and Carcinogens, 1985. 2nd ed. Park Ridge, NJ: Noyes Data Corporation, 1985., p. 464]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": Whenever medical surveillance is indicated, in particular when exposure to a carcinogen has occurred, ad hoc decisions should be taken concerning ... /cytogenetic and/or other/ tests that might become useful or mandatory. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 23]**PEER REVIEWED**
... No biologic monitoring techniques exist at present, either for the reliable determineation of formaldehyde levels in tissue or for the determination of formaldehyde adducts formed with macromolecules. Techniques are under development for nonspecific monitoring of exposure through periodic assessment of chromosome damage (micronucleus formation or sister chromatid exchange frequency) in workers exposed to formaldehyde.
[Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 868]**PEER REVIEWED**
Preemployment baseline data should be recorded for the respiratory tract, liver, and skin condition of any worker who will be exposed to formaldehyde. Thereafter, periodic monitoring should be conducted to detect symptoms of pulmonary or skin sensitization or effects on the liver.
[Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1217]**PEER REVIEWED**
The assessment of formaldehyde exposure can be accomplished through measurement of the metabolite formic acid. Formic acid is also an endogenously produced substance formed by the degradation of glycine. There was no information in the literature that showed a correlation between urinary formic acid levels & formaldehyde exposure levels. This measurement is also a poor indicator of the extent of formaldehyde absorption, due to the high endogenous levels of formic acid. Urine Reference Ranges: Normal- normal population level: 21 mg/l (endogenously produced formic acid); Exposed- not established; Toxic- not established.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 714]**PEER REVIEWED**
Respiratory Symptom Questionnaires: Questionnaires published by the American Thoracic Society (ATS) & the British Medical Research Council have proven useful for identifying people with chronic bronchitis. Certain pulmonary function tests such as the FEV1 have been found to be better predictors of chronic airflow obstruction.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 716]**PEER REVIEWED**
Chest Radiography: Chest radiographs are widely used to assess pulmonary disease. They are useful for detecting early lung cancer in asymptomatic people, & especially for detecting peripheral tumors such as adenocarcinomas. However, even though OSHA mandates this test for exposure to some toxicants such asbestos, experts' views on the risk-to-benefit ratio in detection of pulmonary disease conflict, so routine annual chest x-rays are not recommended for all people.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 716]**PEER REVIEWED**
Pulmonary Function Tests: The tests that have been found to be practical for population monitoring include: Spirometry & expiratory flow-volume curves; Determination of lung volumes; Diffusing capacity for carbon monoxide; Single-breath nitrogen washout; Inhalation challenge tests; Serial measurements of peak expiratory flow; Exercise testing.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 717]**PEER REVIEWED**
Urine Albumin: Albuminuria has been shown to be a specific marker of glomerular dysfunction. Tubular damage, however, can also result in increased levels of albumin in the urine.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 715]**PEER REVIEWED**
Urinary Beta-2-Microglobulin &/or Retinal Binding Protein: Measurements for the presence of either of these low molecular weight proteins are useful in detection of early impairment of proximal tubular function. However, beta-2-microglobulin is unstable at urinary pH <6, & may degrade in the bladder prior to collection & subsequent neutralization of the urine sample. Measurement of retinal binding protein appears to be a better marker for early tubular dysfunction due to its stability in the urine subsequent to collection & analysis. However, retinal binding protein is produced in the liver & not a constitutive protein of the kidney, so that its presence in the kidney provides only indirect evidence of tubular damage.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 715]**PEER REVIEWED**
Urinary Enzyme N-Acetylglucosaminidase: This lysosomal enzyme has shown promise in assessment of subclinical nephrotoxic injury. This enzyme is not normally filtered at the glomerulus due to its high molecular weight. In the absence of glomerular injury, this enzyme will be detected in the urine as a result of leakage or exocytosis from damaged, stimulated, or exfoliated renal cells. The sensitivity of measurement for this enzyme has not been thoroughly studied, but it's usefulness has shown some promise. However, this enzyme is unstable at urinary pH >8, which could diminish the sensitivity of the measurement due to enzyme degradation.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 716]**PEER REVIEWED**
DNA-Protein Crosslinks: Measurement of DNA-protein crosslinks in white blood cells may be a useful test for assessing formaldehyde exposure. In addition, measurement of these crosslinks in other formaldehyde sensitive tissues, such as the upper respiratory tract, may be a useful indicator of formaldehyde exposure. However, other toxicants may cause similar crosslinks, so that the specificity of this test for assessing only formaldehyde exposure is questionable.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 715]**PEER REVIEWED**
Routine Urinalysis: Performing a routine urinalysis including parameters such as specific gravity, glucose, & microscopic exam may be useful for assessing renal toxicity.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 716]**PEER REVIEWED**
Urinary Alpha & Pi Isoenzymes of Glutathione S-Transferase: Radio-immunological & Elisa techniques have been developed for quantitation of /alpha/ & /pi/ isoenzymes of glutathione S-transferase, which are constitutive proteins in the kidney. The /alpha/ isoenzyme is located only in the proximal tubule, while the /pi/ isoenzyme is located in the distal convoluted tubule, the loop of Henle, & the collecting ducts of the kidney. Damage to epithelial cell membranes can result in the increased excretion of these isoenzymes in the urine. This test for assessing renal tubular damage appears to have many advantages over other available tests, such as: (1) the /alpha/ & /pi/ isoenzymes are constitutive proteins in the kidney; (2) these isoenzymes are stable in the urine; (3) the test is simple & reproducible; & (4) due to selective localization of the isoenzymes, differential diagnosis of specific tubular damage is possible. In addition, increased levels of these isoenzymes were seen in patients previously exposed to nephrotoxicants where conventional tests for kidney function were normal, indicating a high degree of sensitivity.
[Ryan, R.P., C.E. Terry, S.S. Leffingwell (eds.) Toxicology Desk Reference 5th ed. Volumes 1-2. Taylor & Francis Philadelphia, PA. 2000, p. 716]**PEER REVIEWED**
Populations at Special Risk:
Mean formaldehyde levels are highest in hospital autopsy rooms compared with other commercial settings. /Hospital autopsy workers are possibly exposed/.
[Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1214]**PEER REVIEWED**
Release of /formaldehyde/ vapors in mobile homes has been associated with headache & pulmonary & dermal irritation. /Occupants of mobile homes are possibly exposed/.
[Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1214]**PEER REVIEWED**
Two populations of humans have received considerable attention in the literature as being particularly sensitive to formaldehyde exposure following inhalation and/or dermal routes. The first population is asthmatics, and concern focuses on the changes in lung function parameters that formaldehyde may produce ... . Most of these studies concluded that there is no evidence of incr airway reactivity as a result of formaldehyde exposure in either normal or asthmatic individuals. ... The second population of potential concern is people with dermal sensitization ... Formaldehyde liquid, but neither the gaseous phase nor formalin, is considered to be a dermal sensitizer ... . Anaphylactic reactions have been reported ... . Dermal allergic reactions have also been reported in doctors and nurses exposed to formaldehyde ... as well as in fiberglass workers ... .
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 236 (1999)]**PEER REVIEWED**
Workers in industries where formaldehyde is used or released may receive potentially high exposures. Members of the general population who live in newly constructed homes or homes where pressed wood products have recently been installed may be exposed to high levels of formaldehyde by inhalation for short periods of time until the latent formaldehyde has been released. Exposure in mobile homes are expected to be higher than conventional homes due to their lower rate of air exchange ... . Members of the general population that handle large amt of permanent press fabrics treated with formaldehyde-releasing resins may also receive potentially high exposures. The use of some cosmetics, such as nail hardeners, may result in high short-term exposure.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 311 (1999)]**PEER REVIEWED**
Smokers and persons who live in a home with a cigarette smoker also may be exposed to higher levels of formaldehyde. Environmental tobacco smoke, which is a combination of diluted sidestream smoke released form a cigarette's burning end and mainstream smoke exhaled by an active smoker, can contribute 10-25% (0.1-1 mg/day) of the total average indoor exposure to formadehyde ... .
[DHHS/ATSDR; Toxicological Profile for Formaldehye p. 311 (1999)]**PEER REVIEWED**
Probable Routes of Human Exposure:
... /VAPORS/ GIVEN OFF DURING HOT MOLDING OF SYNTH RESINS (/IS A/ COMMON SOURCE OF EXPOSURE) ... A SURVEY OF 6 FUNERAL HOMES ... REVEALED MEAN CONCN, IN DIFFERENT ESTABLISHMENTS, BETWEEN 0.25 & 1.39 PPM. ... /EXPOSURES ARE ENCOUNTERED/ IN PHENOL-FORMALDEHYDE RESIN MOULDING PLANT ... /FROM WHICH/ CHRONIC AIRWAY OBSTRUCTION LOWERED FORCED EXPIRATORY VOL/FORCED VOL CAPACITY RATIO & EYE, NOSE & THROAT IRRITATION & LOWER RESP TRACT SYMPTOMS /HAVE BEEN OBSERVED/.
[American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices. 5th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1986., p. 276]**PEER REVIEWED**
... /EXPOSURES TO/ FORMALDEHYDE VAPOR EMISSIONS IN PERMANENT-PRESS FABRICS INDUSTRY (8 PLANTS) /HAVE BEEN REPORTED IN WHICH/ CONCN RANGING ... FROM 0.3 TO 2.7 PPM (IN SEWING AREA) WITH AVG OF 0.68 PPM /WERE DETECTED/. COMPLAINTS CONSISTED OF ANNOYING ODOR (ODOR THRESHOLD, BELOW 1.0 PPM), CONSTANT PRICKLING IRRITATION OF MUCOUS MEMBRANES & DISTURBED SLEEP.
[American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices. 5th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1986., p. 276]**PEER REVIEWED**
NIOSH (NOES Survey 1981-1983) has statistically estimated that 1,329,322 workers (441,902 of these are female) are potentially exposed to formaldehyde in the US(1). The NOES Survey does not include farm workers(SRC). Occupational exposure to formaldehyde may occur through inhalation and dermal contact with this compound at workplaces where formaldehyde is produced or used(2). Monitoring data indicate that the general population may be exposed to formaldehyde via inhalation of ambient air, ingestion of food, and dermal contact with cosmetic and aerosol products containing formaldehyde(2).
[(1) NIOSH; National Occupational Exposure Survey (NOES) (1983) (2) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 243 (1995)]**PEER REVIEWED**
Humans are exposed to formaldehyde from a variety of sources. The major source of atmospheric discharge is from combustion processes specifically from auto emissions and also from the photooxidation of hydrocarbons in auto emissions(1,2). Additional exposure to formaldehyde emissions comes from its use as an embalming fluid in anatomy labs, morgues, etc and its use as a fumigant and sterilant(1). Resin treated fabric, rugs, paper, etc and materials such as particle board and plywood which use resin adhesives and foam insulation release formaldehyde which may build up in homes and occupational atmospheres(1,2). Contact with industrial waste water, especially from lumber related operations where formaldehyde is used in adhesives, has resulted in the Pacific Northwest, Northeast, parts of Texas, and lumber areas of the south(1)(SRC). The estimated daily intake of formaldehyde among exposed Finnish workers is 3000 ug, whereas heavily exposed workers (particle-board and glue production, foundry work) is 10,000 ug(3).
[(1) Kitchens JF et al; Investigation of Selected Potential Environmental Contaminants: Formaldehyde p. 22-98 USEPA 560/2-76-009 (1976) (2) National Research Council; Formaldehyde and Other Aldehydes p. 2-1 to 5-96 USEPA 600/6-82-002 (1982) (3) Hemminki K, Vainio H; Human Exposure to Potentially Carcinogenic Compounds. IARC Sci Publ 59: 37-45 (1984)]**PEER REVIEWED**
In a 12-week study of exposure in a gross anatomy lab of a medical school, 44% of breathing room samples and 11% of ambient air samples were >1.0 ppm the ceiling recommended by ACGIH; Half the breathing zone samples were between 0.6-1.0 ppm and the range was 0.3-2.63 ppm(1). A 1976 report estimates that 8000 US workers were potentially exposed to formaldehyde during its production(3). A more recent estimate of the number of exposed workers in industries producing and using formaldehyde and its derivatives range from 1.4-1.75 million(2). Concentrations of formaldehyde in occupational areas dating from the 1960's and early 1970's are: textile plant 0-2.7 ppm, 0.68 ppm avg; garment factory 0.9-2.7 ppm; clothing store 0.9-3.3 ppm; laminating plant 0.04-10 ppm; funeral homes 0.09-5.26 ppm, 0.25-1.39 ppm avg; resin manufacture and paper production 16-30 ppm; paper conditioning 0.9-1.6 ppm; wood processing 31.2 ppm max(2). Concns in occupational settings dating from the late 70's are: textile plants 0.1-0.5 ppm, 0.2 ppm avg; shoe factory 0.9-2.7 ppm, 1.9 ppm avg; particle board plant 0.1-4.9 ppm, 1.15 ppm avg; plywood plant 0.1-1.2 ppm, 0.35 ppm avg; wooden furniture manufacturing plant 0.1-5.4 ppm, 1.35 ppm avg; adhesive plants 0.8-3.5 ppm, 1.75 ppm avg; foundries 0.05-2.0 ppm, 0.6 ppm avg; construction sites 0.5-7.0 ppm, 2.8 ppm avg; hospitals and clinics 0.05-3.5 ppm, 0.7 ppm avg(2). More recent survey results for occupational environments include: fertilizer production 0.2-1.9 ppm; dyestuffs <0.1-5.8 ppm; textile manufacture <0.1-1.4 ppm; resins (foundry) <0.1-5.5 ppm; bronze foundry 0.12-0.8 ppm; iron foundry <0.02-18.3 ppm; treated paper 0.14-0.99 ppm; hospital autopsy room 2.2-7.9 ppm; plywood industry 1.0-2.5 ppm; urea-formaldehyde foam applicators <0.08-2.4 ppm(4).
[(1) Skisak, CM; Amer Ind Hyg Assoc J 44: 948-50 (1983) (2) IARC; Monograph. Some Industrial Chemicals and Dyestuffs 29: 345-89 (1982) (3) National Research Council; Formaldehyde and other Aldehydes p.2-1 to 5-96 USEPA 600/6-82-002 (1982) (4) Bernstein RS et al; Am Ind Hyg Assoc J 45: 778-85 (1984)]**PEER REVIEWED**
Potential occupational exposure to formaldehyde are as follows: agricultural workers, anatomists, beauticians, biologists, bookbinders, botanists, chemical production workers, cosmetic formulators, crease-resistant textile finishers, disinfectant makers, disinfectors, dress-goods shop personnel, electrical insulation makers, embalmers, embalming fluid makers, fireproofers, formaldehyde production workers, formaldehyde resin makers, foundry employees, fumigators, fur processors, furniture makers, glue and adhesive makers, hide preservers, histology technicians (including necropsy and autopsy technicians), ink makers, lacquerers and lacquer makers, medical personnel (including pathologists), mirror manufacturers, paper makers, particle-board makers, photographic film makers, plastic workers, plywood makers, rubber makers, taxidermists, textiles mordanters and printers, textiles waterproofers, varnish workers, wood preservers(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 225 (1995)]**PEER REVIEWED**
The avg concn of formaldehyde in workroom air in formaldehyde and resin manufacturing plants ranged from 0.1-14.2 mg/cu m(1). The avg concn of formaldehyde in workroom air of plywood mills, particle-board mills, furniture factories, other wood product and paper mills ranged from 0.08-7.4 mg/cu m(1). The avg concn of formaldehyde in workroom air in textile mills and garment factories ranged from 0.1 to 1.9 mg/cu m(1). The avg concn of formaldehyde in workroom air in foundries and other industrial facilities ranged from 0.04 to 38.2 mg/cu m(1). The avg concn of formaldehyde in workroom air in mortuaries, hospitals, and laboratories ranged from 0.05 to 4.2 mg/cu m(1). The avg concn of formaldehyde in workroom air in building sites, agriculture, forestry, and misc other activities ranged from <0.1 to 4.3 mg/cu m(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 226-41 (1995)]**PEER REVIEWED**
Cigarette smoke and products of combustion contain formaldehyde(1). Cigarette smoke contains 15 to 20 mg formaldehyde per cigarette(1). Avg formaldehyde exposure from passive smoking is between 0.23 to 0.27 ppm(1). A 'pack-a-day' smoker may inhale as much as 0.4-2.0 mg formaldehyde(1).
[(1) Bingham E et al, eds; Patty's Toxicology. 5th ed. NY, NY: John Wiley & Sons Inc. 5: 980-3 (2001)]**PEER REVIEWED**
Several studies have been conducted to determine exposure of students in laboratories(1). The concn of formaldehyde in the breathing zone at dissecting tables and in the ambient air in a medical school in the United States was found to be >1.2 mg/cu m in 44% of the breathing zone samples and 11 ambient air samples; 50% of the breathing zone samples contained 0.7-1.2 mg/cu m, with a range of 0.4-3.2 mg/cu m(1). During the 1982-82 academic year, the airborne concn of formaldehyde at a university in the US was 7-16.5 ppm in the laboratory, 1.97-2.62 ppm in the stockroom, and <1 ppm in the public hallway(1). In another study, of 253 samples of air taken during laboratory dissection classes at a university in the US, 97 contained concns above the detection limit of 0.01 mg/cu m; all but four samples had levels <1.2 mg/cu m(1). The avg concn detected was 0.5 mg/cu m(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 226-41 (1995)]**PEER REVIEWED**
Average Daily Intake:
AIR INTAKE: Assume 1 to 100 ug/cu m(1), 20 ug to 2,000 ug formaldehyde(SRC).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 242 (1995)]**PEER REVIEWED**
In Sweden between Dec 1986 to Aug 1987, the mean yearly exposure to formaldehyde from air pollution was 1.2 ug/cu m(1). The estimated daily exposure of the Finnish population to formaldehyde from community air is 100 ug and from the home environment, 1,000 ug(2).
[(1) Bostrom CE et al; Environ Health Perspect 102: 39-47 (1994) (2) Hemminki K, Vainio H; Human Exposure to Potentially Carcinogenic Compounds. IARC Sci Publ 59: 37-45 (1984)]**PEER REVIEWED**
Minimum Fatal Dose Level:
Approximate Minimum Lethal Dose (MLD) (150-lb man): 30 ml
[Arena, J. M. Poisoning: Toxicology, Symptoms, Treatments. Fourth Edition. Springfield, Illinois: Charles C. Thomas, Publisher, 1979., p. 97]**PEER REVIEWED**
Male single oral ingestion 517 mg/kg
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 116 (1999)]**PEER REVIEWED**
Emergency Medical Treatment:
Antidote and Emergency Treatment:
Decontamination: Dilute with milk or water in alert patients as a first aid measure may reduce corrosive effects at the scene. If ingestion has occurred within 1 hr before presentation, gentle gastric aspiration with a soft nasogastric tube may limit systemic absorption. There is little evidence to support the use of activated charcoal to absorb formate or formaldehyde. ... Elimination enhancement: Severe acidosis & deteriorating vital signs are indications for considering dialysis, but the literature does not contain adequate case studies to guide treatment. Aggressive sodium bicarbonate therapy & frequent monitoring of arterial blood gases may be useful. There are no antidotes. Supportive care: 1. Monitor electrolytes, fluids, acid-base, & kidney function closely. 2. Watch for signs of GI hemorrhage & perforation with serial vital signs, abdominal exams, & complete blood counts. 3. Check blood methanol levels & treat accordingly in formalin ingestions. 4. Fibrosis of stomach has required partial gastrectomy in the past.
[Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1217]**PEER REVIEWED**
Irrigate eyes with water. Wash contaminated areas of body with soap and water. Gastric lavage (stomach wash), if swallowed, using 1% ammonium carbonate and followed by saline catharsis. Oxygen, if indicated.
[ITII. Toxic and Hazardous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute, 1988., p. 250]**PEER REVIEWED**
Basic Treatment: Skin- Treated as any burn to prevent allergic contact dermatitis, exposure to formaldehyde or formaldehyde-containing products should be minimized. Inhalation- Patients should be removed from exposure. If symptoms persist, hospitalization may be required. Very high levels (100 ppm) may be lethal. Pulmonary damage may occur. Oral- high concn of formaldehyde may be irritating to the GI tract. Ingestion can result in metabolic responses similar to methanol poisoning. Hemodialysis is efficacious just as in methanol poisoning & should be considered if metabolic acidosis occurs.
[Sullivan, J.B. Jr., G.R. Krieger (eds.). Hazardous Materials Toxicology-Clinical Principles of Environmental Health. Baltimore, MD: Williams and Wilkins, 1992., p. 978]**PEER REVIEWED**
Basic treatment: Establish a patent airway. Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if necessary. Aggressive airway management may be necessary. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Anticipate seizures and treat if necessary ... . Monitor for shock and treat if necessary ... . Monitor for pulmonary edema and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with normal saline during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 ml/kg up to 200 ml of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool. Administer activated charcoal ... . /Aldehydes and related compounds/
[Bronstein, A.C., P.L. Currance; Emergency Care for Hazardous Materials Exposure. 2nd ed. St. Louis, MO. Mosby Lifeline. 1994., p. 234-35]**PEER REVIEWED**
Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious or in respiratory arrest. Intubation should be considered at the first sign of upper airway obstruction caused by edema. Positive pressure ventilation techniques with a bag-valve-mask device may be beneficial. Start an IV with D5W /SRP: "To keep open", minimal flow rate/. Use lactated Ringer's if signs of hypovolemia are present. Watch for signs of fluid overload. Treat seizures with diazepam ... . For hypotension with signs of hypovolemia, administer fluid cautiously. Consider vasopressors if patient is hypotensive with a normal fluid volume. Watch for signs of fluid overload ... . Consider drug therapy for pulmonary edema ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Aldehydes and related compounds/
[Bronstein, A.C., P.L. Currance; Emergency Care for Hazardous Materials Exposure. 2nd ed. St. Louis, MO. Mosby Lifeline. 1994., p. 235]**PEER REVIEWED**
Animal Toxicity Studies:
Evidence for Carcinogenicity:
CLASSIFICATION: B1; probable human carcinogen. BASIS FOR CLASSIFICATION: Based on limited evidence in humans, and sufficient evidence in animals. Human data include nine studies that show statistically significant associations between site-specific respiratory neoplasms and exposure to formaldehyde or formaldehyde-containing products. An increased incidence of nasal squamous cell carcinomas was observed in long-term inhalation studies in rats and in mice. The classification is supported by in vitro genotoxicity data and formaldehyde's structural relationships to other carcinogenic aldehydes such as acetaldehyde. HUMAN CARCINOGENICITY DATA: Limited. ANIMAL CARCINOGENICITY DATA: Sufficient.
[U.S. Environmental Protection Agency's Integrated Risk Information System (IRIS) on Formaldehyde (50-00-0) Available from: http://www.epa.gov/ngispgm3/iris on the Substance File List as of March 15, 2000]**PEER REVIEWED**
A2. A2= Suspected human carcinogen.
[American Conference of Governmental Industrial Hygienists. Threshold Limit Values (TLVs) for Chemical Substances and Physical Agents and Biological Exposure Indices (BEIs) for 1995-1996. Cincinnati, OH: ACGIH, 1995., p. 22]**PEER REVIEWED**
Evaluation: There is limited evidence in humans for the carcinogenicity of formaldehyde. There is sufficient evidence in experimental animals for the carcinogenicity of formaldehyde. Overall evaluation: Formaldehyde is probably carcinogenic to humans (Group 2A).
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V62 336 (1995)]**PEER REVIEWED**
Non-Human Toxicity Excerpts:
INHALATION ... BY ANIMALS CAUSES PROMPT & SEVERE IRRITATION OF EYES & RESP TRACT. ... EDEMA & HEMORRHAGES OF ... LUNG, & SIGNS OF HYPEREMIA & PERIVASCULAR EDEMA IN THE LIVER AND KIDNEYS.
[Patty, F. (ed.). Industrial Hygiene and Toxicology: Volume II: Toxicology. 2nd ed. New York: Interscience Publishers, 1963., p. 1970]**PEER REVIEWED**
PROLONGED EXPOSURE OF RABBITS TO FORMALDEHYDE CAUSED ACID PHOSPHATASE, TWEEN-60-ESTERASE, NAPHTHOL-AS-D-ACETATE-ESTERASE, PROLINE-OXIDASE & HYDROXYPROLINE-2-EPIMERASE ACTIVITIES TO INCREASE & LEUCYL-AMINOPEPTIDASE & BETA-GLUCURONIDASE TO DECREASE. IT INDUCED BRONCHIAL CELL HYPERPLASIA WITH HYPERMUCIGENESIS, EXTRUSION OF BRONCHIAL CELLS, BRONCHIOLAR HYPERMUCIGENESIS, PARCELLARY SQUAMOUS METAPLASIA OR NECROBIOSIS OF EPITHELIA.
[IONESCU J ET AL; MORPHOL EMBRYOL (BUCUR) 24 (3): 232-42 (1978)]**PEER REVIEWED**
CD-1 MICE WERE GIVEN UP TO 185 MG/KG BODY WT FORMALDEHYDE BY GAVAGE ON DAYS 6-15 OF GESTATION. HIGHEST DOSE WAS ... TOXIC TO DAMS, BUT NO EMBRYOTOXICITY OR TERATOGENICITY WAS SEEN WITH ANY DOSE.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V29 366 (1982)]**PEER REVIEWED**
ACUTE ... EFFECTS ... IN RATS ... /& OTHER EXPTL ANIMALS/ TO LOW (LESS THAN 1 PPM) OR MODERATE (10-50 PPM) ... /OF/ VAPOR RESULTED IN INCREASED AIRWAY RESISTANCE, DECR SENSITIVITY OF NASOPALATINE NERVE, IRRITATION OF EYES & OF RESP SYSTEM, & CHANGES IN HYPOTHALAMUS. EXPOSURE TO HIGH DOSES (ABOVE 100 PPM) ... CAUSED SALIVATION, ACUTE DYSPNEA, VOMITING, CRAMPS & DEATH ... .
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V29 364 (1982)]**PEER REVIEWED**
EXPOSURE BY INHALATION FOR UP TO 90 DAYS PRODUCED INTERSTITIAL INFLAMMATION IN LUNGS OF DOGS, RATS, MONKEYS, RABBITS & GUINEA-PIGS. ... HAIR DEPIGMENTATION WAS OBSERVED IN BLACK MICE AT SITE OF SC INJECTION OF 100 UG FORMALDEHYDE. ... MICE TREATED WITH FORMALDEHYDE ON SKIN DEVELOPED SEVERE LIVER DAMAGE.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V29 365 (1982)]**PEER REVIEWED**
GROUPS OF 119-120 MALE & 120 FEMALE FISCHER 344 RATS, 7 WK OF AGE WERE EXPOSED TO 0, 2, 5.6 OR 14.3 PPM (0, 2.5, 6.9, 17.6 MG/CU M) ... GREATER THAN 97.5% PURE VAPOR BY WHOLE-BODY EXPOSURE FOR 6 HR/DAY ON 5 DAYS/WK FOR UP TO 24 MO, FOLLOWED BY 6 MO OBSERVATION PERIOD. ... LIFE-TABLE ANALYSIS OF ... DATA REVEALED SIGNIFICANT INCR (P< 0.0167) IN INCIDENCES OF SQUAMOUS-CELL CARCINOMAS IN /NASAL CAVITY OF RATS/ EXPOSED TO 14.3 PPM FORMALDEHYDE VAPOR; NO OTHER NEOPLASM WAS INCREASED SIGNIFICANTLY. THE INCIDENCE OF A VARIETY OF NON-NEOPLASTIC LESIONS WERE SIGNIFICANTLY INCREASED IN RATS EXPOSED TO FORMALDEHYDE.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V29 361 (1982)]**PEER REVIEWED**
GROUPS OF 6 MALE CYNOMOLGUS MONKEYS ... & 10 MALE & 10 FEMALE SYRIAN GOLDEN HAMSTERS WERE EXPOSED TO 0, 0.2, 1.0 OR 3 PPM (0, 0.24, 1.2 OR 3.7 MG/CU M) FORMALDEHYDE VAPOR (98.8% PURE) FOR 22 HR/DAY ON 7 DAYS/WK FOR 26 WK. SQUAMOUS METAPLASIA OF NASAL TURBINATES WERE EVIDENT IN 6/6 MONKEYS EXPOSED TO 3 PPM & IN 1/6 EXPOSED TO 1 PPM. ... NO EXPOSURE-RELATED EFFECTS WERE DEMONSTRATED IN HAMSTERS.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V29 365 (1982)]**PEER REVIEWED**
REPEATED INHALATION EXPOSURE TO VAPORS AT 15 PPM IN MALE CHARLES RIVER CD RATS & MALE C57BL6/F1 MICE WAS STUDIED. RATS WERE RELATIVELY INSENSITIVE TO IRRITANT ACTION WHILE MICE WERE MORE SENSITIVE, SHOWING COMPARABLE REDUCTION IN TIDAL VOL, BUT GREATER DECR IN RESPIRATORY RATE & MINUTE VOL. CARBON DIOXIDE PRODUCTION AS WELL AS BODY TEMP WERE DECR TO GREATER EXTENT IN MICE THAN IN RATS.
[JAEGER RJ, GEARHART JM; TOXICOLOGY 25 (4): 299-309 (1982)]**PEER REVIEWED**
With Salmonella typhimurium, the minimum concn required to induce 8-azaguanine resistance was 170 uM.
[Goldmacher VS et al; Toxicol Epidemiol Mech (Pap Meet) 173-91 (1983)]**PEER REVIEWED**
15 ppm formaldehyde caused an initial wave of cell replication in the nasal cavity of mice and rats 18 hr after a 6 hr exposure. The /percentage/ of replicating cells remained ... elevated for 3-5 days and then began to decrease. Similar elevations occurred following 3 daily exposures to 6 ppm formaldehyde in rats, but not mice. ...
[Swenberg JA et al; Toxicol Epidemiol Mech (Pap Meet) 225-36 (1983)]**PEER REVIEWED**
... Threshold concn of sensitization effect of /formaldehyde/ in guinea pigs was 0.5 mg/cu m. ... Quantitative changes were seen only in B-lymphocytes, whereas T-lymphocytes were essentially unchanged. At 3 mg/cu m the sensitization effect was seen in all the animals. The T-lymphocytes decreased substantially but B-lymphocytes increased. ...
[Dueva LA; Gig Tr Prof Zabol 8: 20-3 (1983)]**PEER REVIEWED**
... Primary hamster embryo cells were treated by exposure to gaseous formaldehyde or by incorporation into the medium, a dose-related incr in the frequency of SA7 virus transformation was produced. ... Length of chemical treatment and the time interval before subsequent addition of transforming virus was critical, with 2 hr treatment times being most efficient. ... 2.2 ug/ml produced significantly enhanced viral transformation. ...
[Hatch GG et al; Environ Mutagen 5 (1): 49-57 (1983)]**PEER REVIEWED**
... RATS /EXPOSED/ CONTINUOUSLY DURING PREGNANCY TO ... VAPORS (1 MG/CU M) ... /SHOWED/ NO VISIBLE FETAL MALFORMATIONS. ASCORBIC ACID CONTENT OF TREATED FETUSES WAS LOWER THAN CONTROLS BUT BODY WT WAS INCR. FETAL DNA CONTENT WAS DECR & RNA CONTENT WAS INCR.
[Shepard, T.H. Catalog of Teratogenic Agents. 5th ed. Baltimore, MD: The Johns Hopkins University Press, 1986., p. 701]**PEER REVIEWED**
GROUPS OF 100 MALE SPRAGUE-DAWLEY RATS WERE EXPOSED FROM 9 WK OF AGE TO (A) 14.3 PPM (17.44 MG/CU M) FORMALDEHYDE (PURITY UNSPECIFIED) & 10 PPM (16.2 MG/CU M) HYDROGEN CHLORIDE GAS BEFORE DILN IN EXPOSURE CHAMBER TO MAXIMIZE FORMATION OF BIS(CHLOROMETHYL)ETHER; (B) 14.1 PPM (17.2 MG/CU M) FORMALDEHYDE & 9.5 PPM 115.48 MG/CU M) HYDROGEN CHLORIDE NOT MIXED BEFORE INTRODUCTION INTO ... CHAMBER; (C)14.2 PPM (17.32 MG/CU M) FORMALDEHYDE VAPOR ALONE; (D) HYDROGEN CHLORIDE GAS ALONE (10.2 PPM); OR (E) AIR (SHAM-EXPOSED CONTROLS). AFTER ... 382 EXPOSURES OVER ... 588 DAYS (19.4 MO), 10 HISTOLOGICALLY CONFIRMED, GROSSLY VISIBLE NASAL SQUAMOUS-CELL CARCINOMAS WERE OBSERVED IN RATS EXPOSED TO FORMALDEHYDE ALONE; NONE WERE SEEN IN CONTROLS OR IN RATS EXPOSED TO HYDROGEN CHLORIDE ALONE ... COMBINED EXPOSURE TO FORMALDEHYDE & HYDROGEN CHLORIDE DID NOT PRODUCE STATISTICALLY SIGNIFICANT INCR IN INCIDENCE OF NASAL SQUAMOUS-CELL CARCINOMAS OVER THAT OBTAINED WITH FORMALDEHYDE ALONE. ...
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V29 362 (1982)]**PEER REVIEWED**
EXPOSURE OF CULTURED MONKEY KIDNEY CELLS TO 1-16 MMOL ... FOR 15 MIN RESULTED IN FORMATION OF SHORT RNA CHAINS; CONCN EQUAL TO OR GREATER THAN 2 MMOL PRODUCED COMPLETE INHIBITION OF THYMIDINE INCORPORATION & CELL GROWTH. ALMOST COMPLETE REVERSAL OF THESE EFFECTS WERE SEEN WITHIN 24 HR AFTER REMOVAL OF FORMALDEHYDE; SUCH RECOVERY WAS NOT ACCOMPANIED BY UNSCHEDULED DNA SYNTHESIS.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V29 367 (1982)]**PEER REVIEWED**
Addition of aroclor-induced post-mitochondrial supernatant reduced the mutagenicity of formaldehyde in the bacterial cells.
[Goldmacher VS et al; Toxicol Epidemiol Mech (Pap Meet) 173-91 (1983)]**PEER REVIEWED**
DNA-protein crosslinks have been formed in the nasal respiratory mucosa of Fischer-344 rats exposed for 3 hr to selected concentrations of (3)H- and (14)C-formaldehyde. ... In rats depleted of glutathione and exposed to 10 ppm of (3)H-formaldehyde and (14)C-formaldehyde, the (3)H/(14)C ratio of the fraction of the DNA that was crosslinked to proteins was significantly (39 + or - 6%) higher than that of the inhaled gas. This suggests an isotope effect, either on the formation of DNA-protein crosslinks by labeled formaldehyde or on the oxidation of labeled formaldehyde catalyzed by formaldehyde or aldehyde dehydrogenase. These results suggest that the residual (unoxidized) formaldehyde present in the nasal mucosa of rats exposed to (3)H- and (14)C-formaldehyde may be "enriched" in 3-formaldehyde relative to (14)C-formaldehyde which can bind to DNA resulting in an isotope ratio higher than that of the inhaled gas. The isotope effect on the oxidation of (3)formaldehyde and (14)C-formaldehyde suggests that previous estimates of the amount of formaldehyde covalently bound to nasal mucosal DNA may have been too large.
[Heck HD, Casanova M; Toxicol Appl Pharmacol 89 (1): 122-34 (1984)]**PEER REVIEWED**
Glutathione is required for the oxidation of formaldehyde to formate catalyzed by formaldehyde dehydrogenae. The effects of glutathione depletion on the mechanisms of labeling of macromolecules in the rat nasal mucosa and bone marrow by (3)H-formaldehyde and (14)C-formaldehyde were investigated. Male rats were exposed for 3 hr to atmosphere containing (3)H-formaldehyde and (14)C-formaldehyde at concentrations of 0.9, 2,4,6, or 10 ppm, 1 day after a single 3 hr preexposure to the same concentration of unlabeled formaldehyde. Two hours prior to the second exposure, the animals were injected either with phorone (300 mg/kg, ip) or with corn oil. The concentration of nonprotein sulfhydryls in the nasal respiratory mucosa of phorone-injected rats was decreased to 10% of that of corn oil-injected rats. The metabolic incorporation of (3)H-formaldehyde and (14)C-formaldehyde into DNA, RNA and proteins in the respirtory and olfactory mucosa and bone marrow (femur) was significantly decreased, and DNA-protein crosslinking was significantly increased in the respiratory mucosa of phorone injected relative to corn oil injected rats at all formaldehyde concentrations. DNA-protein crosslinks were not detected in the respiratory mucosa of corn oil injected rats at 0.9 ppm. Evidence was obtained for the formation of adducts of formaldehyde with the RNA from the nasal respiratory mucosa of phorone injected rats at concentrations above 0.9 ppm. Covalent binding of formaldehyde to macromolecules in the bone marrow was not detected.
[Casanova M, Heck HD; Toxicol Appl Pharmacol 89 (1): 105-21 (1987)]**PEER REVIEWED**
Fifty-five chemicals, including /formaldehyde/, were evaluated in the Charnoffavlock developmental toxicity screen. All chemicals were administered by gavage to pregnant ICR/SIM mice on gestation day 8-12. The mice were allowed to deliver, & several neonatal growth & viability parameters were measured in the offspring. ... Of the 26 cmpds reported in the literature to be teratogenic or embryotoxic in mice following oral admin, 24 were positive in the developmental toxicity screen. ...
[Seidenberg JM, Becker RA; Teratog Carcinog Mut 7 (1): 17-28 (1987)]**PEER REVIEWED**
... In a plate assay with Salmonella typhimurium strain TA100 in the absence and presence of S9 mix, a weak mutagenic response was observed. Using the pre-incubation method, formaldehyde induced without S9 mix a 1.6-fold and with S9 mix a 2.7-fold increase of revertant numbers over controls.
[Schmid E et al; Mutagen 1 (6): 427-31 (1986)]**PEER REVIEWED**
Poisoning is characterized by severe abdominal pain which may be followed by collapse and death. In less severe cases, acute nephritis with oliguria may develop. Formaldehyde poisoning has been recorded in cattle placed in calving sheds which had been cleaned and disinfected shortly before with a 35% solution of this material, and after drinking from foot-rot treatment baths. The addition of formaldehyde as a preservative to milk has caused intoxiction in calves. The clinical signs recorded included recumbency, abdominal pain, salivation and diarrhea. Postmortem examination revealed severe gastrointestinal tract lesions.
[Humphreys, D.J. Veterinary Toxicology. 3rd ed. London, England: Bailliere Tindell, 1988., p. 192]**PEER REVIEWED**
The use of formalin for the treatment of foot-rot in sheep can give rise to keratinization of the interdigital skin if the solution employed is too concentrated or its application too frequent. In severe cases this may lead to bacterial infection of the feet and result in serious losses.
[Humphreys, D.J. Veterinary Toxicology. 3rd ed. London, England: Bailliere Tindell, 1988., p. 192]**PEER REVIEWED**
Alkaline elution was employed to study DNA damage in Chinese hamster ovary-Kl cells treated with a series of biotic and xenobiotic aldehydes. DNA cross-linking was measured in terms of the reduction in the effect of methyl methanesulphonate on the kinetics of DNA elution and was observed in cells treated with formaldehyde, acetaldehyde, methylglyoxal and malonaldehyde. Propionaldehyde, valeraldehyde, hexanal, and 4-hydroxynonenal produced DNA single strand breaks, or lesions which were converted to breaks in alkali. Both types of DNA damage occurred in cells exposed to malonaldehyde. These findings support the hypothesis of a carcinogenic effect of the aldehydic products (malonaldehyde, methylglyoxal, propionaldehyde, hexanal, 4-hydroxynonenal) released in biomembranes during lipid peroxidation. Acetaldehyde did not cause DNA breaks.
[Marinari UM et al; Cell Biochem Funct 2 (4): 243-8 (1984)]**PEER REVIEWED**
Two groups of 12 male Wistar rats received either 243 ppm of acetaldehyde or 5.7 ppm of formaldehyde for 8 hr a day, 5 days a week during 5 weeks. These levels represent three times the threshold limit values for these substances in Brazilian legislation. The animals were evaluated by pulmonary function tests before and after exposure to the pollutants. The data obtained from these rats were compared with those of 12 controls, housed in identical conditions for the same length of time but breathing normal air. The results showed an increase of the functional residual capacity, residual volume, total lung capacity and respiratory frequency in the rats exposed to acetaldehyde atmosphere. The animals exposed to formaldehyde did not present pulmonary function alterations when compared with the controls. The damage caused by acetaldehyde to the peripheral regions of the lung parenchyma, affecting small airways or altering pulmonary elastic properties, is discussed.
[Saldiva PNH et al; J Appl Toxicol 5 (5): 288-92 (1985)]**PEER REVIEWED**
A 1 year inhalation toxicity study was performed on male albino rats using 0.1, 1.0, or 10 ppm formaldehyde. The nasal mucosa of half the rats was damaged bilaterally by electrocoagulation; 20 to 26 hr after which the rats were subjected to the first 6 hr exposure of formaldehyde. The schedule for the exposures was 6 hr per day, 5 days a week for up to 52 weeks. Decreases in liver glutathione content were noted in rats with damaged noses after 13 weeks exposure. Moderate to severe rhinitis was accompanied by keratinized or nonkeratinized metaplastic respiratory epithelium in rats at the highest exposure levels with or without nasal damage. Growth retardation was observed in the animals with or without a damaged nose after 2 weeks exposure at 10 ppm formaldehyde. At lower exposure levels metaplastic respiratory epithelium occurred only in rats with a damage nasal mucosa, indicating a higher susceptibility of damage mucosa for the irritating and cytotoxic actions of formaldehyde. A more severe basal cell hyperplasia and more severe squamous metaplasia of the respiratory epithelium was noted in rats exposed to 1 ppm formaldehyde and subjected to electroagulation, compared to rats with an undamaged nose and 10 ppm exposure levels. Effects on the olfactory epithelium were exclusively found in animals treated with 10 ppm formaldehyde effects were more posterior in rats with damaged noses, perhaps due to an abnormal air flow pattern in the damaged nose. No adverse effects were seen at 0.1 or 1 ppm in rats with an intact nasal mucosa. The damaged rat nose is more susceptible than the undamaged to the cytotoxicity of formaldehyde, and even at a concentration of 10 ppm formaldehyde has no adverse effects on organs remote from the site of entry in rats with unchanged mucosa.
[Appelman LM et al; J Appl Toxicol 8 (2): 85-90 (1988)]**PEER REVIEWED**
The effects of benzo(a)pyrene & formaldehyde, alone & combined, on cell growth & DNA damage were determined in primary cultures of rat tracheal epithelial cells dissociated from rat tracheas. Cell cultures treated with 25 uM benzo(a)pyrene for 24 hr or 200 uM formaldehyde for 90 min did not have a marked reduction in cell growth. However, their combined treatment reduced cell growth by 60% of control when cultures were exposed to benzo(a)pyrene followed by formaldehyde as well as the reverse order. None of these treatments significantly decreased cell viability as judged by dye exclusion, nor did they enhance cell terminal differentiation as measured by cornified envelope formation. Alkaline elution analysis of DNA damage detected both DNA-protein crosslinks & DNA single strand breaks as a result of formaldehyde treatment, whereas BAP treatment caused only single strand breaks. While formaldehyde induced single strand breaks were repaired within 2 hr, benzo(a)pyrene induced single strand breaks were detected 3 days after treatment. Combined treatment of cell cultures with benzo(a)pyrene followed by formaldehyde resulted in more single strand breaks than was obtained from either agent alone, but less DNA-protein crosslinks than was detected from formaldehyde alone. The increased number of single strand breaks obtained from this combined treatment may be related to the marked enhancement of carcinogenesis observed in earlier in vivo-in vitro studies.
[Cosma GN et al; Mutat Res 201 (1): 161-8 (1988)]**PEER REVIEWED**
The effect of formaldehyde inhalation on total cytochrome p450 in the lungs of Sprague-Dawley rats was assessed after single & repeated exposure to 0, 0.5, 3, & 15 ppm formaldehyde. Whole-body exposures were conducted exposure systems for 6 hr/day, 5 days/wk, for periods of exposure of 1 day, 4 days, 12 wk, or 24 wk. Lung cytochrome p450 were measured 18 hr after the end of exposure at each time point. There were not detectable levels of total lung p450 in any of the rats that received a single 6 hr exposure to all three formaldehyde doses, while control lung p450 levels were similar to that found for 4 day & 12 wk control rats. After 4 days of repeated exposures, however, there was a highly significantly, reproducible, & dose-dependent incr in lung p450 levels relative to controls, with the 0.5, 3, & 15 ppm groups demonstrating 383, 1026, & 1123% of control values, respectively. Lung p450 levels remained elevated all formaldehyde concns through 12 & 24 wk of exposure, although the % difference between exposed & control rats continually dropped throughout the course of long-term repeated exposures. While formaldehyde exposed rats did have decreased total body weight relative to controls, lung microsomal protein & lung weight of nearly all of the formaldehyde exposed rats was not significantly different from the controls. The initial inactivation of lung p450 after a single formaldehyde exposure is apparently a transient phenomenon, with dose-dependent induction of the total p450 levels in the lung as the pattern of response to repeated exposures to inhaled formaldehyde.
[Dallas CE et al; Environ Res 49 (1): 50-9 (1989)]**PEER REVIEWED**
Male Wistar rats were exposed to 0, 10 or 20 ppm formaldehyde vapor for 4, 8, or 13 weeks (6 hr/day; 5 days/week), and were then observed for periods up to 126 weeks. Transient growth retardation occurred in both test groups. Death rate was not noticably affected by formaldehyde. Despite recovery periods of at most 126 weeks, the nasal respiratory and olfactory epithelium of many rats of the 20 ppm group exhibited non-nooplastic histopathological changes. Similar but much less severe changes of the respiratory epithelium were seen in a small number of rats of the 10 ppm group; the olfactory epithelium was not visibly affected in rats of this group. Nasal tumors considered to be induced by formaldehyde were seen only in the 20 ppm group and mainly in rats that had been exposed for 13 weeks, the incidence being 4.5% (6/132). These tumors comprised 3 squamous cell carcinomas, 1 carcinoma in situ and 2 polypoid adenomas, all originating from respiratory epithelium. Rat nasal respiratory epithelium severely damaged by formaldehyde vapor ofter does not regenerate and in some cases develops tumors.
[Feron VJ et al; Cancer Lett 39 (1): 101-11 (1988)]**PEER REVIEWED**
Formaldehyde caused nasal squamous cell carcinomas in the rat following 2 year inhalation exposure. The incidence of this tumor in a historical data base of 16,794 rats was nil, indicating that it is a rare spontaneous tumor. Five different mathematical extrapolation models were applied to the rat nasal tumor data to produce estimates at 10(-4) risk (the size of the historical data base) of between 3.232 and 0.003 ppm formaldehyde depending on the model and choice of maximum likelihood estimate or lower confidence limit values.
[Brown LP; Regul Toxicol Pharmacol 10 (2): 196-200 (1989)]**PEER REVIEWED**
The effects of formaldehyde on mammalian respiratory ciliary function were studied in-vitro. Tracheal rings from New Zealand white rabbits were incubated with 16, 33, or 66 ug/cu m formaldehyde for up to 60 minutes. Formaldehyde induced dose & time dependent decreases in the areas of ciliary activity & ciliary best frequency. The inhibition of ciliary function was reversible, but the times for recovery increased with increasing formaldehyde concn. Porcine tracheal rings were incubated with up to 66 ug/cu m formaldehyde for 60 min followed by up to 65 min recovery. The number of extractable active cilia (ciliary axonemes) was determined. Formaldehyde decreased the number of extractable ciliary axonemes & associated ATPase activity in a dose & time dependent manner. The inhibitory effects were reversible.
[Hastie AT et al; Toxicol & Appl Toxicol 102 (2): 282-91 (1990)]**PEER REVIEWED**
The induction of ornithine-decarboxylase activity and DNA synthesis was studied in the glandular stomach mucosa of rats afer gastric intubation of formaldehyde. Male Fischer rats were given doses of formaldehyde ranging from 11 to 110 mg/kg body weight by gastric intubation. The maximum increase in ornithine-decarboxylase activity was a 100 fold increase noted after 16 hours. The maximum increase in DNA synthesis was a 49 fold increase after 16 hours in the pyloric mucosa of the stomach. Even doses lower than 75 mg/kg, formaldehyde induced ornithine-decarboxylase activity and DNA synthesis in the pyloric mucosa. All the glandular stomach carcinogens and tumor promoters examined have been found to induce ornithine-decarboxylase activity and stimulate DNA synthesis in the glandular stomach mucosa. Inductions of ornithine-decarboxylase activity and DNA synthesis are useful markers of possible tumor promoting activity in the glandular stomach mucosa.
[Furihata C et al; Japanese J of Cancer Res 79 (8): 917-20 (1988)]**PEER REVIEWED**
The relative toxicities of formaldehyde and glutaraldehyde to the rat nasal epithelium were determined following intra-nasal instillation of aqueous solutions of these compounds into one nostril of male Fischer 344 (F-344) rats. Lesions identical in appearance to those resulting from acute inhalation exposure to formaldehyde were induced by both compounds in a concentration-dependent manner. While sterile saline and 10 mM glutaraldehyde induced no significant epithelial changes, 20 and 40 mM glutaraldehyde induced extensive lesions in the treated side of the nose. Aldehyde induced lesions included inflammation, epithelial hypertrophy, and squamous metaplasia in association with marked increases (2-8-fold) in labeling index for both compounds. Formaldehyde induced similar lesions but required concentrations of 200 mM or more to elicit a toxic response. Thus, glutaraldehyde is approximately an order of magnitude more toxic to the nasal epithelium than formaldehyde.
[St Clair M BG et al; Toxicol Pathol 18 (3): 353-61 (1990)]**PEER REVIEWED**
Male and female Sprague-Dawley rats of different ages at the start of the experiments (12 day embryos, and 7 and 25 weeks old) were administered formaldehyde in drinking water at different doses (2,500 or 1,500, 1,000, 500, 100, 50, 10, 0 ppm). An increased incidence of leukemias and of gastro-intestinal tumors was observed in formaldehyde treated rats. Gastro-intestinal tumors are exceptionally rare in the rats of the colony used.
[Soffritti M et al; Toxicol Ind Health 5 (5): 699-730 (1989)]**PEER REVIEWED**
The effects of formaldehyde on the respiratory tract were studied in monkeys. Male rhesus monkeys were exposed to 6 ppm formaldehyde 6 hours/day, 5 days/week for 1 or 6 weeks. Histopathological changes induced by formaldehyde included mild degeneration and early squamous metaplasia in the transitional and respiratory epithelium of the nasal passages and the respiratory epithelium of the trachea and bronchi. There was little difference in the severity of the nasal lesions between animals exposed for 1 or 6 weeks; however, the percentage of nasal mucosal epithelial area with lesions was significantly larger in monkeys exposed for 6 weeks. Only minimal histopathological changes occurred in the lower airways. No treatment related effects were seen in the maxillary sinuses or nonrespiratory ortans. Thymidine labeling indices were significantly increased in the respiratory epithelium of the nasal passages at both 1 and 6 weeks. The areas of greatest proliferation corresponded to the areas of the lesions. Labeling indices in the trachea and carina were significantly elevated after 1 week of exposure. They were nonsignificantly elevated in the transitional and olfactory epithelium of the nasasl passages. Formaldehyde induced nasal lesions are more widespread in the monkey than in the rat, and monkeys are more sensitive to the acute and subacute effects of formaldehyde.
[Monticello TM et al; Am J Path 134 (3): 515-27 (1989)]**PEER REVIEWED**
A 28 month inhalation study was carried out in male SPF reared albino Wistar rats to determine the significance of electrocoagulation damage for the induction of nasal tumors by formaldehyde vapor. Male rats with severely damaged or undamaged noses were exposed 6 hours/day, 5 days/week for 28 months to formaldehyde at concentrations of 0.0, 0.1, 1.0, and 10 ppm. Degenerative, inflammatory and hyperplastic changes were noted in the nasal respiratory and olfactory mucosa in rats with intact noses at the highest dose levels. The incidence of formaldehyde induced rhinitis, hyperplasia and metaplasia of the respiratory epithelium, and degeneration and hyperplasia and metaplasia of the olfactory epithelium all occurred in increased numbers in rats exposed to formaldehyde with damage nasal passages. The incidence of nasal tumors in animals with damage nasal mucosa and treated with 10 ppm formaldehyde for 28 months was 29% (17 of 58 rats), while in the group of rats with an intact nasal mucosa exposed to 10 ppm formaldehyde for 28 months, only 1 of 26 (4%) developed a nasal tumor. Increased tumor incidences were not oberved in rats with damaged nasal mucosa exposed to 0.1 or 1.0 ppm formaldehyde for 28 months or to 0.1, 1.0, or 10 ppm formaldehyde for 3 months. The condition of the nasal mucosa is an important factor in the development of nasal tumors among rats exposed to formaldehyde.
[Woutersen RA et al; J Appl Toxicol 9 (1): 39-46 (1989)]**PEER REVIEWED**
Male and female Wistar rats were given formaldehyde solution in their drinking water at concentrations of 0.50, 0.10, 0.02 and 0% for 24 months. Significant decreases in body weight and food and water intake were observed in the 0.50% group of both sexes and all rats in this group died by 24 months. Various non-neoplastic lesions were observed in rats, mostly in the 0.50% group. In this group, erosions and/or ulcers were evident in both the forestomach and glandular stomach. In the forestomach, squamous cell hyperplasia with or without hyperkeratosis and downward growth of basal cells were observed. Glandular hyperplasia of the fundic mucosa was noted along the limiting ridge. A few of such changes of the upper GI tract were seen in the 0.10% group. No toxicological abnormalities were found in 0.02% group of both sexes. There were no significant differences in the incidences of any tumors among groups of both sexes. Based on these findings, the no observable effect level of formaldehyde was 0.02% in the drinking water (10 mg/kg body wt/day).
[Tobe M et al; Toxicol 56 (1): 79-86 (1989)]**PEER REVIEWED**
The effects of intermittent and continuous inhalation exposure to formaldehyde were studied in rats. Male Wistar rats were exposed to 0, 1, or 2 ppm formaldehyde continuously for 8 hours a day, 5 days a week for 13 weeks. Other rats were exposed to 0, 2, or 4 ppm formaldehyde intermittently, for eight 30 minute exposures separated by 30 mintue periods of nonexposure, 5 days a week for 13 weeks. After 13 weeks, the nasal cavity tissues were examined for histopathological changes. Formaldehyde did not significantly affect body weight again. A slight nonsignificant increase in cell turnover was seen after 3 days in rats exposed intermittently to 2 ppm or continuously to 1 ppm formaldehyde. This effect was not seen after 13 weeks. Treatment related histopathological changes were seen only in nasal tissues from rats exposed intermittently to 4 ppm formaldehyde. These consisted of disarrangement, hyperplasia, and squamous metaplasia with or without keratinization of the respiratory epithelium of the septum and nasoturbinates. These changes were not seen in rats exposed continuously to 2 ppm formaldehyde, which produced the same total daily dose as the intermittent 4 ppm exposure group. Under conditions of formaldehyde, exposure concentration, not total dose, determines the severity of the cytotoxic effects.
[Wilmer JWGM et al; Toxicol Letters 47 (3): 287-93 (1989)]**PEER REVIEWED**
Sprague-Dawley rats were exposed to 0, 5, 10, 20 or 40 ppm formaldehyde for 6 hr/day from day 6 to 20 of gestation. On day 21 of gestation, no effect on embryonic or fetal lethality, nor significant alterations in the external, visceral or skeletal appearance of the fetuses were noted in any of the exposed groups. Significant concentrations-related reduction of fetal body weight occurred at 20 & 40 ppm, & at 40 ppm fetal body weights were 20% < those of the controls. Maternal toxicity, indicated by significant reduction in body weight & absolute weight gain, was observed at 40 ppm. Formaldehyde is slightly fetotoxic at 20 ppm. Neither embryolethal nor teratogenic effects were observed following inhalation exposure at levels up to 40 ppm.
[Saillerfait AM et al; Food Chem Toxicol 27 (8): 545-8 (1989)]**PEER REVIEWED**
An acute exposure study /was conducted/ using 4 groups of 12 Wistar male rats each. One of the 4 groups was used as a control; the other 3 were exposed for 6 hr at 10 ppm, 20 ppm, or 30 ppm. In addn to observing behavioral & other responses during the test period, biochemical & hematologic tests were performed on the test animals. Responses of the 10 ppm exposed group did not differ from those of the controls. In the 20 ppm exposed group, a sniffing motion was observed 1 min after the start of exposure, followed by face-washing movements 10 min later. The face-washing movements decreased with increased exposure time. It was observed at the end of the 6 hr exposure that the hair around the penile area had become yellowed. The movements of the 30 ppm exposed group were similar to those observed for the animals exposed at 20 ppm throughout the exposure period except that hair yellowing was seen 2 hr after the start of exposure. Observations of the 20 ppm & 30 ppm exposed groups also included irritation of the nasal mucosal membrane & trachea, a decr in leukocytes & plasma alkaline phosphatase, & an incr in lung alkaline phosphatase activity. The 10 ppm & 30 ppm groups had a decr in the mean corpuscualr volume & mean corpuscular hemoglobin. The 30 ppm exposed group also had a decr in white blood cells.
[American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991., p. 665]**PEER REVIEWED**
84 articles relating to adverse health effects in animals & humans from subchronic exposures to formaldehyde /were reviewed/ & conclude that animal data revealed a qualitative relationship between formaldehyde absorption & hepatotoxicity. These data indicate that exposure to formaldehyde at 3 ppm or less for periods up to 6 months causes adverse effects upon the liver; higher exposure concns for shorter time periods produce similar effects upon the liver. The reviewed data appear to establish a relationship between exposure to formaldehyde & hepatic degeneration, including decreases in the concn of DNA; mottled, discolored appearance; significant incr in weight; nuclear polymorphism; a profusion of binuclear cells around the triads; focal hyperplasia; & dilatation of hepatic veins with some degeneration of liver cells in the center of the lobules. ... Additional research is required in order to define formaldehyde as a potential human hepatotoxin in formaldehyde exposed populations.
[American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991., p. 666]**PEER REVIEWED**
A long-term formaldehyde animal inhalation bioassay in which groups of about 120 males & 120 female Fischer 344 strain rats & B6C3F1 mice were exposed by inhalation at 0, 2.0, 5.6, or 14.3 ppm of formaldehyde gas for 6 hr/day, 5 days/wk for 24 months. The exposure period was followed by up to 6 months of nonexposure. Squamous cell carcinomas were observed in the nasal cavities of 103 rats (52 females & 51 males) & in 2 male mice; all had been exposed at 14.3 ppm of formaldehyde. One male & one female rat exposed at 5.6 ppm of formaldehyde were also found to have squamous cell carcinomas in their nasal cavities. The two squamous cell carcinomas found in mice exposed at 14.3 ppm formaldehyde were not statistically significant in comparison with the incidence in control mice. However, since this type of nasal lesion is rare in mice, these data can be considered to have biological importance. Benign tumors, such as polypid adenomas, were also seen in male rats in /another/ study at all dose levels & in female rats exposed at 2 ppm formaldehyde. The benign tumor incidence was not linear in this study; benign tumor incidence was highest at the 2 ppm exposure & decreased at higher doses. Since benign nasal tumors are rarely found in rats, the formation in the formaldehyde exposed animals may be attributed to the formaldehyde inhalation.
[American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991., p. 667]**PEER REVIEWED**
Formaldehyde induces gene mutation in bacteria, fungi, yeast, & Drosophila larvae as well as in cultured rodent and human cells. In part, these mutations appear to be the consequence of DNA damage. A second mechanism by which formaldehyde may damage the genome is inhibition of DNA repair.
[Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 868]**PEER REVIEWED**
10 rats (strain, age and sex unspecified) were injected subcutaneously once a week for 15 months with 1 ml of a 0.4% aqueous solution of formaldehyde and then observed for life. Spindle-cell sarcomas were found in three rats; two in the skin at the injection site and one in the peritoneal cavity.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V62 292 (1995)]**PEER REVIEWED**
In a study to evaluate the effects of formaldehyde on gastric carcinogenesis induced by oral administration of N-methyl-N'-nitro-N-nitrosoguanidine, two groups of 10 male Wistar rats, seven weeks of age, received tap water for the first eight weeks of the study. During weeks 8-40, one group then received pure water and the other group received 0.5% formaldehyde in the drinking water. Animals still alive at 40 weeks were killed, rats surviving beyond 30 weeks being considered as effective animals for the study. Necropsy was performed on most animals that died and all animals that were killed, and the stomach and other abdominal organs were examined grossly and histologically. Eight of 10 animals that had received formaldehyde in drinking water and none of the controls developed forestomach papillomas (p< 0.01, Fisher's exact test).
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V62 291 (1995)]**PEER REVIEWED**
Two groups of 16 male and 16 female Oslo hairless mice (age unspecified) received topical applications of 200 ul of 1 or 10% formaldehyde in water on the skin of the back twice a week for 60 weeks. All of the animals treated with 10% formaldehyde were necropsied and the brain, lungs, nasal cavities and all tumors of the skin and other organs were examined histologically. Virtually no changes were found in the mice treated with 1% formaldehyde. The higher dose induced slight epidermal hyperplasia and a few skin ulcers. There were no benign or malignant skin tumors or tumors in other organs in either group.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V62 292 (1995)]**PEER REVIEWED**
Repeated exposure to formaldehyde vapors at 40 ppm, 6 hr/day, 5 day/wk for up to 13 wk produced 80% mortality in B6C3F1 mice, whereas mice exposed with the same protocol to 20 ppm showed no mortalities within the exposure period ... . Deaths occurred predominately in the fifth and sixth wk of exposure and were assoc with ataxia, severe body weight depression, and inflammation and metaplasia in the nasal cavity, larynx, trachea, and lungs. Deaths were attributed to occlusive tracheal lesions and/or prominent seropurulent rhinitis ... . In other intermediate duration inhalation bioassays, no exposure-related deaths or early mortalities were found in Wistar rats exposed to up to 20 ppm, 6 hr/day, 5 days/wk for 13 wk ... in F344 rats, Cynomolgus monkeys, or Golden Syrian hamsters exposed up to 2.95 ppm, 22 hr/day, 7 days/wk for 26 wk ... or in Wistar rats exposed to up to 20 ppm, 6 hr/day, 5 days/wk for 4, 8, or 13 wk and subsequently observed for 117 wk without exposure ... .
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 11 (1999)]**PEER REVIEWED**
In chronic inhalation bioassays, incr mortality ... was found in Sprague-Dawley rats exposed to 14.2 ppm formaldehyde, 6 hr/day, 5 days/wk for up to 588 days ... in F344 rats exposed to 5.6 or 14.3 ppm (but not 2 ppm), 6 hr/day, 5 days/wk for up to 24 mo ... in F344 rats exposed to 15 ppm (but not to 0.7, 2, 6, or 10 ppm) 6 hr/day, 5 days/wk for 24 mo ... and in F344 rats exposed to 15 ppm (but not to 0.3 or 2 ppm), 6 hr/day, 5 days/wk for up to 28 mo ... . In general, observations of incr mortality in the rat bioassays occurred after about one yr of exposure and were assoc with the development of nasal squamous cell carcinomas. Golden Syrian hamster exposed to 10 ppm formaldehyde, 5 hr/day, 5 days/wk for life showed a small, but statistically significant, incr in mortality compared with controls, but no incr incidence of nasal tumors and only a minimal (5% versus zero in controls) incr incidence of hyperplasia or metaplasia in the nasal epithelium ... . No exposure-related incr motality was found in B6C3F1 mice exposed to up to 14.3 ppm for 6 hr/day, 5 days/wk for 24 mo ... .
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 12 (1999)]**PEER REVIEWED**
Studies in animals confirm that the upper respiratory tract is a critical target for inhaled formaldehyde and describe exposure-response relationships for upper respiratory tract irritation and epithelial damage in several species. Acute inhalation animal studies show that inhaled formaldehyde, at appropriate exposure concn, damages epithelial tissue in specific regions of the upper respiratory tract in rats, mice, and monkeys ... that formaldehyde is a more potent sensory irritant in mice ... than in rats ... that lung damage from inhaled formaldehyde occurs at higher concn than those only affecting the upper respiratory tract ... that mice are less susceptible to formaldehyde-induced upper respiratory tract epithelial damage than rats ... that rats and monkeys may be equally susceptible to epithelial damage ... but display similar epithelial lesions in different regions of the upper respiratory tract ... and that formaldehyde induces bronchoconstriction and airway hyperreactivity in guinea pigs ... .
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 54 (1999)]**PEER REVIEWED**
Results from intermediate-duration inhalation studies with rats ... Rhesus monkeys .. Cynomolgus monkeys ... mice ... and hamsters ... indicate that the nasal epithelium is the most sensitive target of inhaled formaldehyde. The studies support the hypothesis that mice and hamsters are less sensitive than rats and monkeys to formaldehyde-induced nasal damage ... show that formaldehyde-induced damage to the upper respiratory tract epithelium (hyperplasia and squamous cell metaplasia) has a wider regional distribution in Rhesus monkeys than in rats ... show that site-specific nasal lesions in both monkeys and rats corresponded to regions with high rates of cellular proliferation ... indicate that damage to the respiratory epithelium is more concn-dependent than duration-dependent ... and show that concn of DNA-protein cross links are correlated with regional sites of formaldehyde-induced epithelial damage in the nose of rats ... .
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 57 (1999)]**PEER REVIEWED**
Chronic-duration exposures in inhaled formaldehyde have also been studied in rats, mice, and hamsters. In rats exposed to concn < or = 15 ppm, formaldehyde-induced effects were restricted to nonneoplastic and neoplastic lesions found primarily in anterior regions of the nasal epithelium, posterior to the vestibule ... . Nonneoplastic damage to rat nasal epithelium occurred at concn as low as 2 ppm, 6 hr/day, 5 days, wk ... whereas significantly incr incidences of neoplastic lesions (squamous cell carcinomas, squamous cell papillomas or polyploid adenomas) were found in rats generally at concn greater than 6 ppm ... . Nonneoplastic damage to upper respiratory tract epithelium has also been observed in mice exposed to > or = 5.6 ppm, 6 hr/day, 5 days/wk for 2 yr ... and in hamsters exposed to 10 ppm, 5 hr/day, 5 days/wk for life ... . Nasal tumors similar to those found in mice exposed to 14.3 ppm for 2 hr ... but were not found in formaldehyde-exposed hamsters ... .
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 62 (1999)]**PEER REVIEWED**
Studies in laboratory animals have also demonstrated that formaldehyde can be genotoxic in some cells after inhalation exposure. ... exposed male Sprague-Dawley rats to formaldehyde concn of 0, 0.5, 3, and 15 ppm, by inhalation for 6 hr/day for 5 days. The rats were sacrificed, and their pulmonary macrophages and bone marrow cells were harvested and analyzed ... . ... An incr in chromosomal abnormalities in pulmonary macrophages, predominantly chromatid breaks, was observed in the 15 ppm group (7.5 versus 3.4% for controls) after 5 days of exposure.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 87 (1999)]**PEER REVIEWED**
... admin 20, 40 or 90 mg/kg/day formaldehyde 5 days/wk for 4 wk to male Wistar rats by gavage. Incr absolute and relative lymph node weights were observed beginning at 40 mg/kg/day. Antibody production was assayed by measurement of total blood IgG and IgM, a hemagglutination assay, a plaque-forming cell assay, and by measurement of IgM production in spleen cells. Only the hemagglutination assay showed a significant effect; the combined IgG and IgM titers were significantly lower than controls at 20 mg/kg/day and above, although individual IgM and IgG titers were only significantly different from controls at 40 and 80 mg/kg/day.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 145 (1999)]**PEER REVIEWED**
Albino guinea pigs (Hartley strain) were treated with 0.1 ml of various dilutions of formalin (1, 3, and 10% formalin; approximately equivalent to 0.4, 1.2, and 4% formaldehyde) to demarcated test sites, and the formalin soln was gently rubbed into the skin with a cotton-tipped applicator ... . An unexposed control site and a vehicle control were used in each series. The sites were left unoccluded and the treatments were repeated once daily immediately after skin-fold measurements. Each site was examined prior to skin-fold measurements for the presence of erythema, edema, fissuring, and scaling. From a mean of 10 sites, erythema appeared on day 2 (4%), day 5 (1.2%), and day 6 (0.4%). Increased skin-fold thickness was statistically significant on day 3 (4%), day 7 (1.2%), and day 9 (0.04%) after daily treatment with various concn of formaldehyde.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 160 (1999)]**PEER REVIEWED**
Increased cell replication occurs as a result of the cytotoxic effects of formaldehyde on the nasal mucosa. Morphological changes (acute degeneration, swelling, formation of "dense bodies", & vacuoles in epithelial cells) were described in the respiratory epithelium of rats after a single 6 hr exposure to 18 mg formaldehyde/cu m. When such exposure was repeated 3-5 times, ulceration was observed in the respiratory epithelium in most experimental animals. After a 9-day exposure, reparative hyperplasia & metaplasia were found. At 7.2 mg/cu m, hyperplasia & slight degenerative changes were still detected. In contrast, morphological changes could not be proved at 0.6 & 2.5 mg formaldehyde/cu m.
[WHO; Environ Health Criteria 89: Formaldehyde p.134 (1989)]**PEER REVIEWED**
Non-Human Toxicity Values:
LD50 Rat oral 800 mg/kg
[ITII. Toxic and Hazardous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute, 1988., p. 249]**PEER REVIEWED**
LD50 Rat sc 420 mg/kg
[ITII. Toxic and Hazardous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute, 1988., p. 249]**PEER REVIEWED**
LD50 Mouse sc 300 mg/kg
[ITII. Toxic and Hazardous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute, 1988., p. 249]**PEER REVIEWED**
LD50 Guinea pig oral 260 mg/kg
[ITII. Toxic and Hazardous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute, 1988., p. 249]**PEER REVIEWED**
LD50 Rabbit percutaneous 270 mg/kg /Formalin/
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 525]**PEER REVIEWED**
LC50 Rat inhalation 0.82 mg/l (1/2 hour)
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 525]**PEER REVIEWED**
LC50 Rat inhalation 0.48 mg/l (4 hr)
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 525]**PEER REVIEWED**
LC50 Mouse inhalation 0.414 mg/l (4 hr)
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 525]**PEER REVIEWED**
LD50 Rat oral 100 mg/kg
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
LD50 Rat iv 87 mg/kg
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
LD50 Mouse oral 42 mg/kg
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
LC50 Mouse inhalation 400 mg/cu m/2 hr
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
Ecotoxicity Values:
LC50 Striped bass larvae 10 mg/l/48-96 hr; static bioassay.
[Environmental Canada; Tech Info for Problem Spills: Formaldehyde p.67 (1985)]**PEER REVIEWED**
Median lethal dose Rainbow trout (Salmo gairdneri) 50 mg/l/48 hr. /Conditions of bioassay not specified/
[Environment Canada; Tech Info for Problem Spills: Formaldehyde p.68 (1985)]**PEER REVIEWED**
LC50 Flounder 100-300 mg/l/48 hr (aerated salt water) /Conditions of bioassay not specified/
[Environment Canada; Tech Info for Problem Spills: Formaldehyde p.70 (1985)]**PEER REVIEWED**
LC50 Rainbow trout (Salmo gairdnerii) (green egg) 565 mg/l/96 hr static bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Rainbow trout (Salmo gairdnerii) (eyed egg) 198 mg/l/96 hr static bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Rainbow trout (Salmo gairdnerii) (sac larvae) 89.5 mg/l/96 hr static bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Rainbow trout (Salmo gairdnerii) fingerlings 61.9 mg/l/96 hr static bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Rainbow trout (Salmo gairdnerii) 440 mg/l/96 hr static bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Rainbow trout (Salmo gairdnerii) 214 mg/l/24 hr static bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Rainbow trout (Salmo gairdnerii) 118 ul/l/96 hr flow-through bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Atlantic salmon (Salmo salar) 173 ul/l/96 hr flow-through bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Lake trout (Salvelinus namaycush) 100 ul/l/96 hr flow-through bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Black bullhead (Ameiurus melas or Ictalurus melas) 62.1 ul/l/96 hr flow-through bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Channel catfish (Ictalurus punctatus) 65.8 ul/l/96 hr flow-through bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Green sunfish (Lepomis cyanellus) 173 ul/l/96 hr flow-through bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Bluegill (Lepomis macrochirus) 100 ul/l/96 hr flow-through bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Smallmouth bass (Micropterus dolomieui) 136 ul/l/96 hr flow-through bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Largemouth bass (Micropterus salmoides) 143 ul/l/96 hr flow-through bioassay
[Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 680]**PEER REVIEWED**
LC50 Pimephales promelas (fathead minnow) 24.1 mg/l/96 hr (confidence limit 22.6 - 25.7 mg/l), flow-through bioassay with measured concentrations, 21.7 deg C, dissolved oxygen 7.4 mg/l, hardness 50.8 mg/l calcium carbonate, alkalinity 37.0 mg/l calcium carbonate, and pH 6.8.
[Geiger D.L., D.J. Call, L.T. Brooke. (eds.). Acute Toxicities of Organic Chemicals to Fathead Minnows (Pimephales- Promelas). Vol. V. Superior WI: University of Wisconsin-Superior, 1990., p. 31]**PEER REVIEWED**
TSCA Test Submissions:
Chronic toxicity and oncogenicity were evaluated in male and female Fischer 344 rats (120/sex/dose level, 240/sex controls) exposed to formaldehyde by inhalation at 0, 2, 6 or 15 ppm for 6 hrs/day, 5 days/week, for 24 months. A total of 95 confirmed cases of nasal squamous cell carcinoma were observed in rats exposed to the highest dose level, 3 cases were observed in rats exposed to 6 ppm, and no cases were observed at the 2 ppm dose level or in controls. Further results from this study were not reported in this progress report.
[Chemical Industry Institute of Toxicology; Progress Report on CIIT Formaldehyde Studies. (1980), EPA Old Document No. 44004, Fiche No. OTS0507060 ]**UNREVIEWED**
Chronic toxicity and oncogenicity were evaluated in male and female B6C3F1 mice (120/sex/dose level, 240/sex controls) exposed to formaldehyde by inhalation at 0, 2, 6 or 15 ppm for 6 hrs/day, 5 days/week, for 24 months. A total of 2 confirmed cases of nasal squamous cell carcinoma were observed in mice exposed to the highest dose level and no cases were observed at the 2 or 6 ppm dose levels or in controls. Further results from this study were not reported in this progress report.
[Chemical Industry Institute of Toxicology; Progress Report on CIIT Formaldehyde Studies. (1980), EPA Old Document No. 44004, Fiche No. OTS0507060 ]**UNREVIEWED**
The effects of acute oral exposure to formaldehyde by gavage in male Wistar rats (20 in control group (water), 5/treated group, number of treated groups not reported) were determined. Formaldehyde (100 or 200 mg/kg) was administered in a single dose and the rats were necropsied on the 11th day following dosing. There were differences between treated and control animals at the highest does level in the following: increase in sperm head count, and a highly significant increase in the percentage of abnormal sperm heads, including straight heads (i.e. no hook), excessive curvature of heads, folded, coiled, thin or amorphous heads. There were no significant differences between treated and control animals in the following: clinical observations, histopathology of the testes, and testes weights.
[Shell Oil Co.; The Effects of Acute Exposure of Dimethoxyethyl Phthalate, Glycerol Alpha-monochlorohydrin, Epichlorohydrin, Formaldehyde and Methylmethanesulfonate Upon Testicular Sperm in the Rat. (1982), EPA Document No. 878210077, Fiche No. OTS206200 ]**UNREVIEWED**
Metabolism/Pharmacokinetics:
Metabolism/Metabolites:
RAPID OXIDN OF FORMALDEHYDE INTO FORMATE FOLLOWED BY FURTHER OXIDN TO CARBON DIOXIDE TAKES PLACE PRINCIPALLY IN ERYTHROCYTES & LIVER.
[The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 339]**PEER REVIEWED**
WHEN FEMALE RATS WERE ADMIN (14)C-FORMALDEHYDE IP AT DOSE LEVEL OF 70 MG/KG, 82% OF DOSE WAS EXPIRED AS (14)CARBON DIOXIDE & 13-14% WAS EXCRETED VIA KIDNEYS IN FORM OF METHIONINE, SERINE, & FORMALDEHYDE-CYSTEINE ADDUCT.
[The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 340]**PEER REVIEWED**
Rats injected ip with 0.26 mg/kg (14)C-labeled formaldehyde ... excreted approx 22% of this dose in the urine over 5 days. Formic acid & a thiazolidine-4-carboxylic acid derivative were identified in urine as formaldehyde metabolites.
[Hemminki K; Chem-Biol Interact 48 (2): 243-8 (1984)]**PEER REVIEWED**
SHORTLY AFTER IV INJECTION OF 35 MG/KG FORMALDEHYDE, INTO DOGS, THERE WAS NO INCR IN PLASMA FORMALDEHYDE CONCN, BUT BIG INCR IN FORMIC ACID CONCN. ... THE RATE OF FORMALDEHYDE OXIDN IS COMPARABLE IN SEVERAL SPECIES OF MAMMALS ...
[The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 339]**PEER REVIEWED**
A novel modification for urinary formic acid analysis was developed in order to gain experience in the biological monitoring of farmers exposed to the acid vapors in silage making. It appeared that the farmers excreted varying amounts of acid before the actual silage making period, but all showed increased excretion rates up to 15 hr after the exposures. The data indicated that formic acid may have a long biological half-life possibly causing an accumulation of the acid in the body. This might constitute unappreciated toxicological hazard, as the acid is an inhibitor of oxygen metabolism.
[Liesivuori J; Ann Occup Hyg 30 (3): 329-34 (1986)]**PEER REVIEWED**
The effect of deuterium substitution on the metab of formaldehyde & formate to carbon dioxide in vivo was examined. 4 groups of male Sprague-Dawley rats were injected ip with (14)C labeled formaldehyde, formaldehyde-d2, sodium formate, or sodium formate-d at doses of 0.67 mmol/kg. Similar rates of labeled carbon dioxide exhalation were observed for the 4 groups of animals, the cumulative excretion of (14)Carbon dioxide in breath reaching 68-71% of the theoretical value 12 hr after injection in all cases. Plots of amount remaining to be excreted showed that the metab was biexponential, with half-lives of approx 0.4 & 3 hr for the two phases for each of the 4 compounds. ...
[Keefer LK et al; Drug Metals Dispos 15 (3): 300-4 (1987)]**PEER REVIEWED**
Homogenates of respiratory & olfactory tissue from the rat nasal cavity were examined for their capacity to catalyze the NAD(+)-dependant oxidation of formaldehyde (in the presence & absence of glutathione) & of acetaldehyde. Both aldehydes were oxidized efficiently by nasal mucosal homogenates, & formaldehyde dehydrogenase & aldehyde dehydrogenase were tentatively identified in both tissue samples. At least 2 isoenzymes of aldehyde dehydrogenase differing either with respect to their apparent Km & max values with acetaldehyde as substrate, were found in the nasal mucosa, one of which may catalyze the oxidation of both formaldehyde & acetaldehyde. ... Repeated exposures of rats to formaldehyde (15 ppm, 6 hr/day, 10 days) or to acetaldehyde (1500 ppm, 6 hr/day, 5 days) did not substantially affect the specific activities of formaldehyde dehydrogenase & aldehyde dehydrogenase in nasal mucosal homogenates. Glutathione is a cofactor for formaldehyde dehydrogenase; the concn of nonprotein sulfhydryls in respiratory mucosal homogenates was approx 2.8 uM/g & was not changed significantly by repeated exposures to formaldehyde (15 ppm, 6 hr/day, 9 days). These data indicate that the rat nasal mucosa, which is the major target site for both aldehydes in inhalation toxicity studies, can metabolize both formaldehyde & acetaldehyde, & that the specific activities of formaldehyde & aldehyde dehydrogenase in homogenates of the nasal mucosa are essentially unchanged following repeated exposures to toxic concns of either cmpnd.
[Casanova-Schmitz M et al; Biochem Pharmacol 33 (7): 1137-42 (1984)]**PEER REVIEWED**
The movement of blood formaldehyde in rabbits that were intoxicated with methanol has been investigated. When methanol alone was admin to rabbits orally, formaldehyde could not be detected in the blood. Further, in an experiment on the metab of methanol in vitro, formaldehyde was not detected in specimen samples but formate was. In contrast, when methanol was orally admin to rabbits that had been pretreated with diethyldithiocarbamate, an aldehyde dehydrogenase inhibitor, 17 to 33 microM of formaldehyde were detected in the blood 4 hr later. However, formaldehyde was not detected in the blood when methanol was orally admin to rabbits that had been pretreated with pyrazole, & alcohol dehydrogenase, inhibitor. After rabbits were given an iv admin of formaldehyde, & on the addition of formaldehyde to a rabbit liver homogenate & blood, the formaldehyde in both instances was metabolized rapidly. Formaldehyde that was not metabolized within 10-15 min, however, bound to the tissue proteins. Formaldehyde was seen to be rapidly metabolized to formate without accumulating in the blood or binding to the tissue proteins.
[Matsumoto K et al; Nippon Hoigaku Zasshi 44 (3): 205-11 (1990)]**PEER REVIEWED**
Formaldehyde is a normal metabolite of the body involved in methylation reactions through the tetrafolate mechanism; normal blood levels of formaldehyde in humans & animals are approx 2.5 ppm (2.5 mg/l). Formaldehyde is rapidly metabolized with a half-life in the blood of approx 1.5 min. This half-life is based primarily on primate data although available human data are consistent with this observation of a very short half-life. Data from other species suggest that the half-life of formaldehyde is fairly similar in many species. Formaldehyde's normal blood levels & short half-life, as well as the assumption that the levels of water soluble formaldehyde in the blood are in equilibrium with the body fluids pool, lead to a calculation that an adult human body normally produces & metabolizes (detoxify or utilizes) over 50,000 ug of endogenous formaldehyde/day. Formaldehyde is either converted to carbon dioxide by the formate pathway & then exhaled or incorporated into the one carbon pool. Radioactivity following exposure to 14C-formaldehyde is found throughout the body & supports the concept of rapid incorporation & metab.
[Sullivan, J.B. Jr., G.R. Krieger (eds.). Hazardous Materials Toxicology-Clinical Principles of Environmental Health. Baltimore, MD: Williams and Wilkins, 1992., p. 974]**PEER REVIEWED**
Formaldehyde may be formed endogenously after contact with xenobiotics; 18 chemicals have been shown to be metabolized by the nasal microsomes of rats to produce formaldehyde. Formaldehyde is a normal metabolite in mammalian systems. It is rapidly metabolized to formate which is partially incorporated via normal metabolic pathways into the one-carbon pool of the body or further oxidized to carbon dioxide. Formaldehyde also reacts with proteins & nucleic acids; it reacts with single-strand DNA, but not with double-strand DNA. This link is reversible. Only formaldehyde cross-links of DNA & protein are stable. ... The oxidation of absorbed formaldehyde to formic acid is catalyzed by several enzymes. The most important enzyme is the NAD-dependent formaldehyde dehydrogenase, which requires reduced glutathione (GSH) as a cofactor. Thus, exogenous formaldehyde becomes a source of the so-called one-carbon pool in intermediary metab. ... There are at least 7 enzymes that catalyze the oxidation of formaldehyde in animal tissues, namely aldehyde dehydrogenase, xanthinoxidase, catalase, peroxidase, glycerinaldehyde-3-phosphate dehydrogenase, aldehyde oxidase, & a specific DPN-dependent formaldehyde dehydrogenase.
[WHO; Environ Health Criteria 89: Formaldehyde p.81 (1989)]**PEER REVIEWED**
Incubation of formaldehyde with human nasal mucus in vitro resulted in the reversible formation of protein adducts, primarily with albumin, suggesting that a portion of the inhaled formaldehyde is retained in the mucous blanket. No adducts were found in high relative-molecular-mass glycoproteins. Absorbed formaldehyde may react with nucleophiles (e.g., amino and sulfhydryl groups) at or near the absorption site, or it can be oxidized to formate and exhaled as carbon dioxide or incorporated into biological macromolecules via tetrahydrofolate-dependent one-carbon biosynthetic pathways.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V62 299 (1995)]**PEER REVIEWED**
Several of the urinary excretion products of formaldehyde in rats have been identified after intraperitoneal administration of (14)C-formaldehyde. After injecting Wistar rats with 0.26 mg/kg body weight, ... formate and a sulfur-containing metabolite (thought to be a derivative of thiazolidine-4-carboxylic acid) and products presumed to result from one-carbon metabolism /were detected/. Thiazolidine-4-carboxylate, which is formed via the nonenzymatic condensation of formaldehyde with cysteine, was not detected in urine.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V62 299 (1995)]**PEER REVIEWED**
The toxicokinetics of formaldehyde after inhalation, oral, or dermal exposure has been reported in several species by many investigators. The toxicokinetics in all of the animals studied is similar across species lines. Formaldehyde is an essential metabolic intermediate in all cells. It is produced during the normal metabolism of serine, glycine, methionine, and choline and also by the demethylation of N-, S-, and O-methyl compounds. After oxidation of formaldehyde to formate, the carbon atom is further oxidized to carbon dioxide (CO2) or incorporated into purines, thymidine, and amino acids via tetra-hydrofolate-dependent one-carbon biosynthetic pathways. Exogenous formaldehyde appears to be readily absorbed from the respiratory and GI tracts, but poorly absorbed following dermal application. Formaldehyde is metabolized to formate by the enzyme formaldehyde dehydrogenase; this appears to take place at the initial site of contact. Being normal components of intermediary metabolism, neither formaldehyde nor formate are stored to any significant extent in any tissue of the body. Formate is either excreted in the urine (primarily as formic acid), incorporated into other cellular molecules, or oxidized to carbon dioxide and exhaled.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 166 (1999)]**PEER REVIEWED**
Formaldehyde is rapidly metabolized and storage is not a factor in its toxicity. The metabolism of formaldehyde to formate ... takes place in all of the tissues of the body as a consequence of endogenous formation of formaldehyde, and the formate is quickly removed by the supporting blood supply ... . Formaldehyde dehydrogenase (FDH) is the major metabolic enzyme involved in the metabolism of formaldehyde in all of the tissues studies; it is widely distributed in animal tissues, particularly in the rat nasal mucosa, and is specific for the glutathione adduct of formaldehyde. If formaldehyde is not metabolized by FDH, then it can form cross linkages between proteins, between protein and single-stranded DNA ... or enter the 1 carbon intermediary metabolic pool by initially binding to tetrahydrofolate ... . Several enzymes can catalyze the reaction that oxidizes formaldehyde to formic acid ... however, FDH is the primary enzyme that performs this function and is specific for formaldehyde ... . Endogenous of exogenous formaldehyde enters the FDH metabolic pathway and is eliminated from the body as metabolites, primarily as formate or CO2. Formaldehyde dehydrogenase activity does not incr ... in response to formaldehyde exposure ... thus no incr in metabolism occurs.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 176 (1999)]**PEER REVIEWED**
Absorption, Distribution & Excretion:
... ABSORBED FROM ALIMENTARY & RESP TRACTS.
[Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 179]**PEER REVIEWED**
IN RATS & MICE ADMIN (14)C-FORMALDEHYDE INTRAGASTRICALLY, 40% OF DOSE ... /WAS/ EXPIRED AS CARBON DIOXIDE, 10% /WAS/ EXCRETED IN URINE & 1% IN FECES AFTER 12 HR; CARCASSES CONTAINED 20% AFTER 24 HR & 10% AFTER 4 DAYS. WHEN FEMALE RATS WERE ADMIN (14)C-FORMALDEHYDE IP AT DOSE LEVEL OF 70 MG/KG, 82% OF DOSE WAS EXPIRED AS (14)CARBON DIOXIDE & 13-14% WAS EXCRETED VIA KIDNEYS ... .
[The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 340]**PEER REVIEWED**
Less than 1% of the /skin/ applied dose of (14)C /as formaldehyde/ was excreted or concn in the major organs of the monkey. Approx 10 times this amt was found in the rat and guinea pig excreta and internal organs. ... The skin of the monkey was much less permeable to formaldehyde than that of rodents. A significant proportion ... was found after 72 hr at the site of application, in the skin and fur, and ... for rodents ... in the remaining carcass.
[Jeffcoat AR et al; Chem Ind Inst Toxicol Conf on Formaldehyde Toxicol p.38-50 (1983)]**PEER REVIEWED**
Airborne (14)C-labeled formaldehyde was primarily absorbed in the upper respiratory tract of rats, leading to a very high radioactive concn in the nasal mucosa. ...
[Heck HD et al; Chem Ind Inst Toxicol, Conf on Formaldehyde Toxicol p.26-37 (1983)]**PEER REVIEWED**
The effect of subchronic exposure to formaldehyde on blood formaldehyde concentrations was studied in monkeys. Young adult Rhesus monkeys were exposed to 0 or 6.00 ppm formaldehyde vapor 6 hours per day, 5 days per week for 4 weeks. Blood samples were obtained at 7 minutes and at 45 hours after the last exposure. The average blood formaldehyde concentrations obtained 7 minutes and 45 hours after exposure were 1.84 and 2.04 ug/g, respectively. The average blood formaldehyde concentraton in the controls was 2.42 ug/g. None of the concentrations were statistically different from each other. Subchronic exposure to a relatively high concentration of formaldehyde does not significantly increase the blood formaldehyde concentration of Rhesus monkeys. This result agrees with those of previous studies in rats and humans. Because formaldehyde is rapidly metabolized it does not accumulate in the blood or produce toxic effects at distant sites. The concentration of endogenous formaldehyde in the blood of Rhesus monkeys is similar to that of humans.
[Casanova M et al; Food and Chem Toxicol 26 (8): 715-6 (1988)]**PEER REVIEWED**
Formaldehyde is readily absorbed from the respiratory & oral tract, & to a much lesser degree from the skin. Formaldehyde is the simplest aldehyde & reacts readily with macromolecules such as proteins & nucleic acids. Inhalation exposure has been reported to result in almost complete absorption. Dermal absorption due to contact with formaldehyde-containing materials such as textiles, perma-press clothing, cosmetics, or other materials is of low order of magnitude. ... Formaldehyde is normally converted & excreted as carbon dioxide in the air, as formic acid in the urine, or as one of many breakdown products from one carbon pool metab. Because of rapid absorption by both the oral & inhalation route & the rapid metab, little or no formaldehyde is excreted unmetabolized. Rats exposed to 14C-formaldehyde by inhalation had 40% of the radiolabel excreted in the air & 20% in the urine & feces; 40% remained in the carcass.
[Sullivan, J.B. Jr., G.R. Krieger (eds.). Hazardous Materials Toxicology-Clinical Principles of Environmental Health. Baltimore, MD: Williams and Wilkins, 1992., p. 974]**PEER REVIEWED**
Formaldehyde is absorbed rapidly and almost completely from the rodent intestinal tract. In rats, about 40% of an oral dose of (14)C-formaldehyde (7 mg/kg) was eliminated as (14)C-carbon dioxide within 12 hours, while 10% was excreted in the urine and 1% in the feces. A substantial portion of the radioactivity remained in the carcass as products of metabolic incorporation.
[IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)., p. V62 296 (1995)]**PEER REVIEWED**
Formaldehyde vapors are readily absorbed from the respiratory tract. Due to rapid metabolism to formate, little, if any, intact formaldehyde can be found in the blood of humans or animals exposed to formaldehyde. Formaldehyde is also readily absorbed from the GI tract and meets with the same metabolic fate as formaldehyde after inhalation exposure. The studies available in the open literature suggest that very little formaldehyde is absorbed via the dermal route. In all cases, absorption appears to be limited to cell layers immediately adjacent tot eh point of contact. Entry of formaldehyde into the blood (i.e., systemic absorption) occurs to a very limited extent, if at all.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 166 (1999)]**PEER REVIEWED**
Biological Half-Life:
... IN SEVERAL SPECIES ... FORMALDEHYDE HAS HALF-LIFE OF ONLY 1 MIN; BUT THE HALF-LIFE FOR FORMIC ACID IS SPECIES DEPENDENT.
[The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 339]**PEER REVIEWED**
Formaldehyde is rapidly metabolized with a half-life in the blood of approx 1.5 min. This half-life is based primarily on primate data although available human data are consistent with this observation of a very short half-life. Data from other species suggest that the half-life of formaldehyde is fairly similar in many species.
[Sullivan, J.B. Jr., G.R. Krieger (eds.). Hazardous Materials Toxicology-Clinical Principles of Environmental Health. Baltimore, MD: Williams and Wilkins, 1992., p. 974]**PEER REVIEWED**
Mechanism of Action:
The exact mechanism by which formaldehyde exerts its irritant, corrosive, and cytotoxic effects is not known. Aldehydes as a group are reactive chemicals with a highly electronegative oxygen atom and less electronegative atoms of carbon(s), and hence have a substantial dipole moment. The carbonyl atom is the electrophilic site of these type of molecules, making it react easily with nucleophilic sites on cell membranes and in body tissues and fluids such as the amino groups in protein and DNA ... .
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 191 (1999)]**PEER REVIEWED**
... formaldehyde readily combines with free, unprotonated amino groups of amino acids to yield hydroxymethyl amino acid derivatives and a proton (H+), which is believed to be related to its germicidal properties. Higher concn will precipitate protein ... . Either one of these mechanistic properties or perhaps other unknown properties may be responsible for the irritation effects seen with formaldehyde exposure. It is probable that formaldehyde toxicity occurs when intracellular levels saturate formaldehyde dehydrogenase activity, overwhelming the natural protection against formaldehyde, and allowing the unmetabolized intact molecule to exert its effects locally.
[DHHS/ATSDR; Toxicological Profile for Formaldehye p. 192 (1999)]**PEER REVIEWED**
Interactions:
MICE EXPOSED TO 11 COMBINATIONS OF ACROLEIN-FORMALDEHYDE; RESPIRATORY RATE MONITORED & RESULTS INDICATE COMPETITIVE AGONISM BETWEEN ACROLEIN & FORMALDEHYDE.
[KANE LE, ALARIE Y; AM IND HYG ASSOC J 39 (4): 270-4 (1978)]**PEER REVIEWED**
/IN GUINEA PIGS/ 1 HR EXPOSURE TO CONCN OF 0.3 PPM & ABOVE PRODUCED INCR IN PULMONARY FLOW RESISTANCE ACCOMPANIED BY LESSER DECREASE IN COMPLIANCE. ... THE RESPONSE ... POTENTIATED BY SIMULTANEOUS ADMIN OF ... SODIUM CHLORIDE AEROSOL OF SUBMICRON PARTICLES. THE VALUES FOR PULMONARY RESISTANCE REMAINED ABOVE PREEXPOSURE LEVELS FOR 1 HR AFTER THE END OF EXPOSURE WHEN THE GAS-AEROSOL COMBINATION WAS USED. THIS PROLONGED RESPONSE ... SUGGEST THAT THE POTENTIATION IS BROUGHT ABOUT BY THE ATTACHMENT OF FORMALDEHYDE TO THE PARTICLES TO FORM AN IRRITANT AEROSOL. THIS ... IS FURTHER SUPPORTED BY FACT THAT WHEN 3, 10 & 30 MG/CU M CONCN OF SODIUM CHLORIDE WERE USED, THE POTENTIATION INCR WITH INCREASING CONCENTRATION OF PARTICLES. THE RESPONSE TO A GIVEN CONCN OF FORMALDEHYDE PLUS AEROSOL BREATHED BY NOSE WAS GREATER THAN THE RESPONSE TO THE GAS ALONE BREATHED THROUGH A TREACHEAL CANNULA.
[Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 867]**PEER REVIEWED**
C3H/10T1/2 cells were treated with N-methyl-N'-nitro-N-nitrosoguanidine then repeatedly exposed to /formaldehyde/ (0.1-2.0 ug/ml). Exposure of N-methyl-N'-nitro-N-nitrosoguanidine initiated cultures to /formaldehyde/ of 0.5 or 1.0 ug/ml in a variety of treatment regimens resulted in focus formation in up to 9% of the treated dishes. Transformed foci were observed in < 2% of the cultures treated N-methyl-N'-nitro-N-nitrosoguanidine or /formaldehyde/ alone. Formaldehyde ... appears to be only a weak tumor promotor for C3H/10T1/2 cell transformation.
[Frazelle JH et al; Cancer Res 43 (7): 3236-9 (1983)]**PEER REVIEWED**
A study was performed on four groups of Sprague-Dawley rats: one exposed to wood dust (25 mg/cu m), another to formaldehyde (12.4 ppm) and a third to both wood dust and formaldehyde; the fourth group served a control group. After 104 weeks of exposure the nose and lungs were examined histologically. One well differentiated squamous cell carcinoma was found in the formaldehyde group. Squamous cell metaplasia was found significantly more often among the formaldehyde exposed rats. Squamous cell metaplasia with dysplasia was most frequently observed, however, in the group exposed to both formaldehyde and wood dust. There were also significantly more rats with pulmonary emphysema in the groups exposed to wood dust than in the other groups.
[Holmstrom M et al; Acta Otolaryngol 108 (3-4): 274-83 (1989)]**PEER REVIEWED**
The combined effects on the nasal epithelium of mixtures of ozone and formaldehyde at cytotoxic and noncytotoxic concentrations were examined. Male Wistar rats were exposed by inhalation during 22 hr/day for 3 consecutive days to 0.3, 1.0 or 3.0 ppm formaldehyde or to 0.2, 0.4, or 0.8 ppm ozone, or they were sham exposed to clean air. Treatment related histopathological nasal changes, such as dissarrangement, loss of cilia, and hyper/metaplasia of the epithelium were seen at 0.2, 0.4, and 0.8 ppm ozone and at 3 ppm formaldehyde. Simultaneous exposure to both materials did not noticeable affect type, degree, and size the microscopic nasal lesions.
[Reuzel P GJ et al; J Toxicol Environ Health 29 (3): 279-92 (1990)]**PEER REVIEWED**
In cultured human bronchial fibroblasts exposed to the carcinogen N-methyl-N-nitrosourea (NMU) in combination with formaldehyde, formaldehyde was observed to inhibit repair of alkylation of DNA at the O6 guanine position induced by NMU. Whether formaldehyde enhances the effects of other DNA-damaging agents has not yet been evaluated.
[Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 868]**PEER REVIEWED**
The sensory irritant effect of formaldehyde at 1.2 mg/cu m was shown to decr when the chemical pyridine was injected into the chanber; such sensory interactions occur in environmentally realistic situations.
[WHO; Environ Health Criteria 89: Formaldehyde p.138 (1989)]**PEER REVIEWED**
... experiments with mice ... and guinea pigs ... indicate that exposure to low levels of formaldehyde enhances allergic responses to intranasal admin of ovalbumin and suggest the possibility of formaldehyde facilitation of allergic responses to other respiratory allergens.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 236 (1999)]**PEER REVIEWED**
Pharmacology:
Therapeutic Uses:
Disinfectants; Fixatives
[National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)]**PEER REVIEWED**
DESENSITIZING TEETH /SOLN, USP/ /FORMER USE/
[Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 971]**PEER REVIEWED**
MEDICATION (VET): FOR VARIOUS SKIN DISEASES OF LARGE ANIMALS & DEMODECTIC MANGE IN DOG /SOLN, USP/
[Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1091]**PEER REVIEWED**
MEDICATION (VET): ANTISEPTICS, FUMIGANT, HAS BEEN USED IN TYMPANY, DIARRHEA, MASTITIS, PNEUMONIA, INTERNAL BLEEDING.
[Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 604]**PEER REVIEWED**
MEDICATION (VET): ... In cattle ... foot-rot treatment baths ... Treatment of foot-rot in sheep ... .
[Humphreys, D.J. Veterinary Toxicology. 3rd ed. London, England: Bailliere Tindell, 1988., p. 192]**PEER REVIEWED**
Interactions:
MICE EXPOSED TO 11 COMBINATIONS OF ACROLEIN-FORMALDEHYDE; RESPIRATORY RATE MONITORED & RESULTS INDICATE COMPETITIVE AGONISM BETWEEN ACROLEIN & FORMALDEHYDE.
[KANE LE, ALARIE Y; AM IND HYG ASSOC J 39 (4): 270-4 (1978)]**PEER REVIEWED**
/IN GUINEA PIGS/ 1 HR EXPOSURE TO CONCN OF 0.3 PPM & ABOVE PRODUCED INCR IN PULMONARY FLOW RESISTANCE ACCOMPANIED BY LESSER DECREASE IN COMPLIANCE. ... THE RESPONSE ... POTENTIATED BY SIMULTANEOUS ADMIN OF ... SODIUM CHLORIDE AEROSOL OF SUBMICRON PARTICLES. THE VALUES FOR PULMONARY RESISTANCE REMAINED ABOVE PREEXPOSURE LEVELS FOR 1 HR AFTER THE END OF EXPOSURE WHEN THE GAS-AEROSOL COMBINATION WAS USED. THIS PROLONGED RESPONSE ... SUGGEST THAT THE POTENTIATION IS BROUGHT ABOUT BY THE ATTACHMENT OF FORMALDEHYDE TO THE PARTICLES TO FORM AN IRRITANT AEROSOL. THIS ... IS FURTHER SUPPORTED BY FACT THAT WHEN 3, 10 & 30 MG/CU M CONCN OF SODIUM CHLORIDE WERE USED, THE POTENTIATION INCR WITH INCREASING CONCENTRATION OF PARTICLES. THE RESPONSE TO A GIVEN CONCN OF FORMALDEHYDE PLUS AEROSOL BREATHED BY NOSE WAS GREATER THAN THE RESPONSE TO THE GAS ALONE BREATHED THROUGH A TREACHEAL CANNULA.
[Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 867]**PEER REVIEWED**
C3H/10T1/2 cells were treated with N-methyl-N'-nitro-N-nitrosoguanidine then repeatedly exposed to /formaldehyde/ (0.1-2.0 ug/ml). Exposure of N-methyl-N'-nitro-N-nitrosoguanidine initiated cultures to /formaldehyde/ of 0.5 or 1.0 ug/ml in a variety of treatment regimens resulted in focus formation in up to 9% of the treated dishes. Transformed foci were observed in < 2% of the cultures treated N-methyl-N'-nitro-N-nitrosoguanidine or /formaldehyde/ alone. Formaldehyde ... appears to be only a weak tumor promotor for C3H/10T1/2 cell transformation.
[Frazelle JH et al; Cancer Res 43 (7): 3236-9 (1983)]**PEER REVIEWED**
A study was performed on four groups of Sprague-Dawley rats: one exposed to wood dust (25 mg/cu m), another to formaldehyde (12.4 ppm) and a third to both wood dust and formaldehyde; the fourth group served a control group. After 104 weeks of exposure the nose and lungs were examined histologically. One well differentiated squamous cell carcinoma was found in the formaldehyde group. Squamous cell metaplasia was found significantly more often among the formaldehyde exposed rats. Squamous cell metaplasia with dysplasia was most frequently observed, however, in the group exposed to both formaldehyde and wood dust. There were also significantly more rats with pulmonary emphysema in the groups exposed to wood dust than in the other groups.
[Holmstrom M et al; Acta Otolaryngol 108 (3-4): 274-83 (1989)]**PEER REVIEWED**
The combined effects on the nasal epithelium of mixtures of ozone and formaldehyde at cytotoxic and noncytotoxic concentrations were examined. Male Wistar rats were exposed by inhalation during 22 hr/day for 3 consecutive days to 0.3, 1.0 or 3.0 ppm formaldehyde or to 0.2, 0.4, or 0.8 ppm ozone, or they were sham exposed to clean air. Treatment related histopathological nasal changes, such as dissarrangement, loss of cilia, and hyper/metaplasia of the epithelium were seen at 0.2, 0.4, and 0.8 ppm ozone and at 3 ppm formaldehyde. Simultaneous exposure to both materials did not noticeable affect type, degree, and size the microscopic nasal lesions.
[Reuzel P GJ et al; J Toxicol Environ Health 29 (3): 279-92 (1990)]**PEER REVIEWED**
In cultured human bronchial fibroblasts exposed to the carcinogen N-methyl-N-nitrosourea (NMU) in combination with formaldehyde, formaldehyde was observed to inhibit repair of alkylation of DNA at the O6 guanine position induced by NMU. Whether formaldehyde enhances the effects of other DNA-damaging agents has not yet been evaluated.
[Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 868]**PEER REVIEWED**
The sensory irritant effect of formaldehyde at 1.2 mg/cu m was shown to decr when the chemical pyridine was injected into the chanber; such sensory interactions occur in environmentally realistic situations.
[WHO; Environ Health Criteria 89: Formaldehyde p.138 (1989)]**PEER REVIEWED**
... experiments with mice ... and guinea pigs ... indicate that exposure to low levels of formaldehyde enhances allergic responses to intranasal admin of ovalbumin and suggest the possibility of formaldehyde facilitation of allergic responses to other respiratory allergens.
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 236 (1999)]**PEER REVIEWED**
Minimum Fatal Dose Level:
Approximate Minimum Lethal Dose (MLD) (150-lb man): 30 ml
[Arena, J. M. Poisoning: Toxicology, Symptoms, Treatments. Fourth Edition. Springfield, Illinois: Charles C. Thomas, Publisher, 1979., p. 97]**PEER REVIEWED**
Male single oral ingestion 517 mg/kg
[DHHS/ATSDR; Toxicological Profile for Formaldehyde p. 116 (1999)]**PEER REVIEWED**
Environmental Fate & Exposure:
Environmental Fate/Exposure Summary:
Formaldehyde is ubiquitous in the environment; it is an important endogenous chemical that occurs in most life forms, including humans. It is formed naturally in the troposphere during the oxidation of hydrocarbons. Formaldehyde's production and use in the manufacture of resins, disinfectants, preservatives, and a variety of other chemicals may result in its release to the environment through various waste streams. Formaldehyde's production and use as a fertilizer results in its direct release to the environment. If released to air, formaldehyde will exist solely as a gas in the ambient atmosphere. Gas-phase formaldehyde will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is 41 hrs. Formaldehyde absorbs ultraviolet radiation at wavelengths of >360 nm. Formaldehyde has a half-life of 6 hrs in simulated sunlight. If released to soil, formaldehyde is expected to have very high mobility based upon an estimated Koc of 37. Volatilization from moist soil surfaces is not expected to be an important fate process based upon a Henry's Law constant of 3.4X10-7 atm-cu m/mole. Formaldehyde volatilizes from dry soil surfaces because it is a gas. If released into water, formaldehyde is not expected to adsorb to suspended solids and sediment based upon the estimated Koc. Formaldehyde readily biodegrades under both aerobic and anaerobic conditions in the environment. Formaldehyde in aqueous effluent was degraded by activated sludge and sewage in 48-72 hr. In a die-away test using water from a stagnant lake, degradation was complete in 30 and 40 hrs under aerobic and anaerobic conditions, respectively. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's Henry's Law constant. Experiments performed on a variety of fish and shrimp show no bioconcentration of formaldehyde. Formaldehyde is not expected to undergo hydrolysis in the environment because of the lack of hydrolyzable functional groups. Occupational exposure to formaldehyde may occur through inhalation and dermal contact with this compound at workplaces where formaldehyde is produced or used. Monitoring data indicate that the general population is exposed to formaldehyde via inhalation of ambient air, ingestion of food, and dermal contact with cosmetic and aerosol products containing formaldehyde. Concns of formaldehyde in outdoor and indoor air range from 1 to 20 ug/cu m and 25 to 100 ug/cu m, respectively. (SRC)
**PEER REVIEWED**
Probable Routes of Human Exposure:
... /VAPORS/ GIVEN OFF DURING HOT MOLDING OF SYNTH RESINS (/IS A/ COMMON SOURCE OF EXPOSURE) ... A SURVEY OF 6 FUNERAL HOMES ... REVEALED MEAN CONCN, IN DIFFERENT ESTABLISHMENTS, BETWEEN 0.25 & 1.39 PPM. ... /EXPOSURES ARE ENCOUNTERED/ IN PHENOL-FORMALDEHYDE RESIN MOULDING PLANT ... /FROM WHICH/ CHRONIC AIRWAY OBSTRUCTION LOWERED FORCED EXPIRATORY VOL/FORCED VOL CAPACITY RATIO & EYE, NOSE & THROAT IRRITATION & LOWER RESP TRACT SYMPTOMS /HAVE BEEN OBSERVED/.
[American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices. 5th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1986., p. 276]**PEER REVIEWED**
... /EXPOSURES TO/ FORMALDEHYDE VAPOR EMISSIONS IN PERMANENT-PRESS FABRICS INDUSTRY (8 PLANTS) /HAVE BEEN REPORTED IN WHICH/ CONCN RANGING ... FROM 0.3 TO 2.7 PPM (IN SEWING AREA) WITH AVG OF 0.68 PPM /WERE DETECTED/. COMPLAINTS CONSISTED OF ANNOYING ODOR (ODOR THRESHOLD, BELOW 1.0 PPM), CONSTANT PRICKLING IRRITATION OF MUCOUS MEMBRANES & DISTURBED SLEEP.
[American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices. 5th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1986., p. 276]**PEER REVIEWED**
NIOSH (NOES Survey 1981-1983) has statistically estimated that 1,329,322 workers (441,902 of these are female) are potentially exposed to formaldehyde in the US(1). The NOES Survey does not include farm workers(SRC). Occupational exposure to formaldehyde may occur through inhalation and dermal contact with this compound at workplaces where formaldehyde is produced or used(2). Monitoring data indicate that the general population may be exposed to formaldehyde via inhalation of ambient air, ingestion of food, and dermal contact with cosmetic and aerosol products containing formaldehyde(2).
[(1) NIOSH; National Occupational Exposure Survey (NOES) (1983) (2) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 243 (1995)]**PEER REVIEWED**
Humans are exposed to formaldehyde from a variety of sources. The major source of atmospheric discharge is from combustion processes specifically from auto emissions and also from the photooxidation of hydrocarbons in auto emissions(1,2). Additional exposure to formaldehyde emissions comes from its use as an embalming fluid in anatomy labs, morgues, etc and its use as a fumigant and sterilant(1). Resin treated fabric, rugs, paper, etc and materials such as particle board and plywood which use resin adhesives and foam insulation release formaldehyde which may build up in homes and occupational atmospheres(1,2). Contact with industrial waste water, especially from lumber related operations where formaldehyde is used in adhesives, has resulted in the Pacific Northwest, Northeast, parts of Texas, and lumber areas of the south(1)(SRC). The estimated daily intake of formaldehyde among exposed Finnish workers is 3000 ug, whereas heavily exposed workers (particle-board and glue production, foundry work) is 10,000 ug(3).
[(1) Kitchens JF et al; Investigation of Selected Potential Environmental Contaminants: Formaldehyde p. 22-98 USEPA 560/2-76-009 (1976) (2) National Research Council; Formaldehyde and Other Aldehydes p. 2-1 to 5-96 USEPA 600/6-82-002 (1982) (3) Hemminki K, Vainio H; Human Exposure to Potentially Carcinogenic Compounds. IARC Sci Publ 59: 37-45 (1984)]**PEER REVIEWED**
In a 12-week study of exposure in a gross anatomy lab of a medical school, 44% of breathing room samples and 11% of ambient air samples were >1.0 ppm the ceiling recommended by ACGIH; Half the breathing zone samples were between 0.6-1.0 ppm and the range was 0.3-2.63 ppm(1). A 1976 report estimates that 8000 US workers were potentially exposed to formaldehyde during its production(3). A more recent estimate of the number of exposed workers in industries producing and using formaldehyde and its derivatives range from 1.4-1.75 million(2). Concentrations of formaldehyde in occupational areas dating from the 1960's and early 1970's are: textile plant 0-2.7 ppm, 0.68 ppm avg; garment factory 0.9-2.7 ppm; clothing store 0.9-3.3 ppm; laminating plant 0.04-10 ppm; funeral homes 0.09-5.26 ppm, 0.25-1.39 ppm avg; resin manufacture and paper production 16-30 ppm; paper conditioning 0.9-1.6 ppm; wood processing 31.2 ppm max(2). Concns in occupational settings dating from the late 70's are: textile plants 0.1-0.5 ppm, 0.2 ppm avg; shoe factory 0.9-2.7 ppm, 1.9 ppm avg; particle board plant 0.1-4.9 ppm, 1.15 ppm avg; plywood plant 0.1-1.2 ppm, 0.35 ppm avg; wooden furniture manufacturing plant 0.1-5.4 ppm, 1.35 ppm avg; adhesive plants 0.8-3.5 ppm, 1.75 ppm avg; foundries 0.05-2.0 ppm, 0.6 ppm avg; construction sites 0.5-7.0 ppm, 2.8 ppm avg; hospitals and clinics 0.05-3.5 ppm, 0.7 ppm avg(2). More recent survey results for occupational environments include: fertilizer production 0.2-1.9 ppm; dyestuffs <0.1-5.8 ppm; textile manufacture <0.1-1.4 ppm; resins (foundry) <0.1-5.5 ppm; bronze foundry 0.12-0.8 ppm; iron foundry <0.02-18.3 ppm; treated paper 0.14-0.99 ppm; hospital autopsy room 2.2-7.9 ppm; plywood industry 1.0-2.5 ppm; urea-formaldehyde foam applicators <0.08-2.4 ppm(4).
[(1) Skisak, CM; Amer Ind Hyg Assoc J 44: 948-50 (1983) (2) IARC; Monograph. Some Industrial Chemicals and Dyestuffs 29: 345-89 (1982) (3) National Research Council; Formaldehyde and other Aldehydes p.2-1 to 5-96 USEPA 600/6-82-002 (1982) (4) Bernstein RS et al; Am Ind Hyg Assoc J 45: 778-85 (1984)]**PEER REVIEWED**
Potential occupational exposure to formaldehyde are as follows: agricultural workers, anatomists, beauticians, biologists, bookbinders, botanists, chemical production workers, cosmetic formulators, crease-resistant textile finishers, disinfectant makers, disinfectors, dress-goods shop personnel, electrical insulation makers, embalmers, embalming fluid makers, fireproofers, formaldehyde production workers, formaldehyde resin makers, foundry employees, fumigators, fur processors, furniture makers, glue and adhesive makers, hide preservers, histology technicians (including necropsy and autopsy technicians), ink makers, lacquerers and lacquer makers, medical personnel (including pathologists), mirror manufacturers, paper makers, particle-board makers, photographic film makers, plastic workers, plywood makers, rubber makers, taxidermists, textiles mordanters and printers, textiles waterproofers, varnish workers, wood preservers(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 225 (1995)]**PEER REVIEWED**
The avg concn of formaldehyde in workroom air in formaldehyde and resin manufacturing plants ranged from 0.1-14.2 mg/cu m(1). The avg concn of formaldehyde in workroom air of plywood mills, particle-board mills, furniture factories, other wood product and paper mills ranged from 0.08-7.4 mg/cu m(1). The avg concn of formaldehyde in workroom air in textile mills and garment factories ranged from 0.1 to 1.9 mg/cu m(1). The avg concn of formaldehyde in workroom air in foundries and other industrial facilities ranged from 0.04 to 38.2 mg/cu m(1). The avg concn of formaldehyde in workroom air in mortuaries, hospitals, and laboratories ranged from 0.05 to 4.2 mg/cu m(1). The avg concn of formaldehyde in workroom air in building sites, agriculture, forestry, and misc other activities ranged from <0.1 to 4.3 mg/cu m(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 226-41 (1995)]**PEER REVIEWED**
Cigarette smoke and products of combustion contain formaldehyde(1). Cigarette smoke contains 15 to 20 mg formaldehyde per cigarette(1). Avg formaldehyde exposure from passive smoking is between 0.23 to 0.27 ppm(1). A 'pack-a-day' smoker may inhale as much as 0.4-2.0 mg formaldehyde(1).
[(1) Bingham E et al, eds; Patty's Toxicology. 5th ed. NY, NY: John Wiley & Sons Inc. 5: 980-3 (2001)]**PEER REVIEWED**
Several studies have been conducted to determine exposure of students in laboratories(1). The concn of formaldehyde in the breathing zone at dissecting tables and in the ambient air in a medical school in the United States was found to be >1.2 mg/cu m in 44% of the breathing zone samples and 11 ambient air samples; 50% of the breathing zone samples contained 0.7-1.2 mg/cu m, with a range of 0.4-3.2 mg/cu m(1). During the 1982-82 academic year, the airborne concn of formaldehyde at a university in the US was 7-16.5 ppm in the laboratory, 1.97-2.62 ppm in the stockroom, and <1 ppm in the public hallway(1). In another study, of 253 samples of air taken during laboratory dissection classes at a university in the US, 97 contained concns above the detection limit of 0.01 mg/cu m; all but four samples had levels <1.2 mg/cu m(1). The avg concn detected was 0.5 mg/cu m(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 226-41 (1995)]**PEER REVIEWED**
Average Daily Intake:
AIR INTAKE: Assume 1 to 100 ug/cu m(1), 20 ug to 2,000 ug formaldehyde(SRC).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 242 (1995)]**PEER REVIEWED**
In Sweden between Dec 1986 to Aug 1987, the mean yearly exposure to formaldehyde from air pollution was 1.2 ug/cu m(1). The estimated daily exposure of the Finnish population to formaldehyde from community air is 100 ug and from the home environment, 1,000 ug(2).
[(1) Bostrom CE et al; Environ Health Perspect 102: 39-47 (1994) (2) Hemminki K, Vainio H; Human Exposure to Potentially Carcinogenic Compounds. IARC Sci Publ 59: 37-45 (1984)]**PEER REVIEWED**
Natural Pollution Sources:
Formaldehyde is ubiquitous in the environment; it is an important endogenous chemical that occurs in most life forms, including humans(1). It is formed naturally in the troposphere during the oxidation of hydrocarbons, which react with hydroxyl radicals and ozone to form formaldehyde and other aldehydes, as intermediates in a series of reactions that ultimately lead to the formation of carbon monoxide and carbon dioxide, hydrogen and water(1). Of the hydrocarbons found in the troposphere, methane is the single most important source of formaldehyde(1). Terpenes and isoprene, emitted by foliage, react with hydroxyl radicals, forming formaldehyde as an intermediate product(1). Because of their short half-life, these potentially important sources of formaldehyde are important only in the vicinity of vegetation(1). Formaldehyde is one of the volatile compounds formed in the early stages of decomposition of plant residues in the soil(1). Formaldehyde occurs naturally in fruits and other foods(1). Other sources are forest fires, animal wastes, microbial products of biological systems, and plant volatiles(2,3). Formaldehyde can also be formed in seawater by photochemical processes(4). However, calculations of sea-air exchange indicates that this process is probably a minor source for formaldehyde in the sea(4).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 242 (1995) (2) Graedel TE; Chemical Compounds in the atmosphere. NY, NY Academic Press p. 161 (1978) (3) Kitchens JF et al; Investigation of selected potential environmental contaminants: formaldehyde. Washington DC: USEPA, Off Tox Subst USEPA 560/2-76-009 p. 22-80 (1976) (4) Mopper K, Stahovec WL; Marine Chem 19: 305-21 (1986)]**PEER REVIEWED**
Artificial Pollution Sources:
Formaldehyde's production and use in the manufacture phenol-formaldehyde resins, urea-formaldehyde resins, acetal resins, 1,4-butanediol, melamine resins, pentaerythritol, hexamethylenetetramine, urea-formaldehyde concentrates, methylene diisocyanate(1), ethylene glycol, pentaerythritol, hexamethylenetetramine, and a variety of other chemicals(2), and its use as a disinfectant, biocide, embalming fluid, preservative, reducing agent (eg, in recovery of gold and silver), corrosion inhibitor in oil wells, and industrial sterilant(2) may result in its release to the environment through various waste streams(SRC). Formaldehyde's production and use as an fertilizer(2) results in its direct release to the environment(SRC).
[(1) Gerberich HR, Seaman GC; Kirk-Othmer Encycl Chem Technol. 4th ed. NY, NY: John Wiley and Sons, 11: 944 (1994) (2) Lewis RJ Sr, ed; Hawley's Condensed Chem Dict. 13th ed. NY, NY: John Wiley and Sons Inc, p. 514 (1997)]**PEER REVIEWED**
Environmental Fate:
TERRESTRIAL FATE: Based on a classification scheme(1), an estimated Koc value of 37(SRC), determined from a log Kow of 0.35(2) and a regression-derived equation(3), indicates that formaldehyde is expected to have very high mobility in soil(SRC). Volatilization of formaldehyde from moist soil surfaces is not expected to be an important fate process(SRC) given a Henry's Law constant of 3.4X10-7 atm-cu m/mole(4). Volatilization of formaldehyde from dry soil surfaces because it is a gas(5). Formaldehyde readily biodegrades under both aerobic and anaerobic conditions in the environment but most of these tests have been conducted under aqueous conditions(SRC). Formaldehyde in aqueous effluent was degraded by activated sludge and sewage in 48-72 hr(6-10). In a die-away test using water from a stagnant lake, degradation was complete in 30 and 40 hrs under aerobic and anaerobic conditions, respectively(7).
[(1) Swann RL et al; Res Rev 85: 17-28 (1983) (2) Hansch C et al; Exploring QSAR. Hydrophobic, Electronic, and Steric Constants. ACS Prof Ref Book. Heller SR, consult. ed., Washington, DC: Amer Chem Soc p. 3 (1995) (3) Lyman WJ et al; Handbook of Chemical Property Estimation Methods. Washington, DC: Amer Chem Soc pp. 4-9 (1990) (4) Betterton EA, Hoffmann MR; Environ Sci Technol 22: 1415-8 (1988) (5) Boublik T et al; The vapor pressures of pure substances. Vol. 17. Amsterdam, Netherlands: Elsevier Sci. Publ p. 44 (1984) (6) CITI; Biodegradation and Bioaccumulation Data of Existing Chemicals. Formaldehyde (50-00-0). Available from the Database Query page at http://www.cerij.or.jp/ceri_en/index_e4.shtml as May 8, 2001. (7) Kitchens JF et al; Investigation of selected potential environmental contaminants; formaldehyde. Washington DC: USEPA, Off Tox Subst USEPA 560/2-76-009 p. 99-110 (1976) (8) Hatfield R; Ind Eng Chem 49: 192-6 (1957) (9) Heukelekian H, Rand MC; J Water Pollut Control Assoc 29: 1040-53 (1955) (10) Verschueren K; Handbook of environmental data on organic chemicals 4th ed. NY, NY: John Wiley and Sons, p. 1170-4 (2001)]**PEER REVIEWED**
AQUATIC FATE: Based on a classification scheme(1), an estimated Koc value of 37(SRC), determined from a log Kow of 0.35(2) and a regression-derived equation(3), indicates that formaldehyde is not expected to adsorb to suspended solids and sediment(SRC). Volatilization from water surfaces is not expected(3) based upon a Henry's Law constant of 3.4X10-7 atm-cu m/mole(4). According to a classification scheme(5), a BCF of 3(SRC), from its log Kow(2) and a regression-derived equation(6), suggests the potential for bioconcentration in aquatic organisms is low(SRC). Formaldehyde readily biodegrades under both aerobic and anaerobic conditions in the environment(SRC). Formaldehyde in aqueous effluent was degraded by activated sludge and sewage in 48-72 hr(7-11). In a die-away test using water from a stagnant lake, degradation was complete in 30 and 40 hrs under aerobic and anaerobic conditions, respectively(8).
[(1) Swann RL et al; Res Rev 85: 17-28 (1983) (2) Hansch C et al; Exploring QSAR. Hydrophobic, Electronic, and Steric Constants. ACS Prof Ref Book. Heller SR, consult. ed., Washington, DC: Amer Chem Soc p. 3 (1995) (3) Lyman WJ et al; Handbook of Chemical Property Estimation Methods. Washington, DC: Amer Chem Soc pp. 4-9, 15-1 to 15-29 (1990) (4) Betterton EA, Hoffmann MR; Environ Sci Technol 22: 1415-8 (1988) (5) Franke C et al; Chemosphere 29: 1501-14 (1994) (6) Meylan WM et al; Environ Toxicol Chem 18: 664-72 (1999) (7) CITI; Biodegradation and Bioaccumulation Data of Existing Chemicals. Formaldehyde (50-00-0). Available from the Database Query page at http://www.cerij.or.jp/ceri_en/index_e4.shtml as May 8, 2001. (8) Kitchens JF et al; Investigation of selected potential environmental contaminants; formaldehyde. Washington DC: USEPA, Off Tox Subst USEPA 560/2-76-009 p. 99-110 (1976) (9) Hatfield R; Ind Eng Chem 49: 192-6 (1957) (10) Heukelekian H, Rand MC; J Water Pollut Control Assoc 29: 1040-53 (1955) (11) Verschueren K; Handbook of environmental data on organic chemicals 4th ed. NY, NY: John Wiley and Sons, p. 1170-4 (2001)]**PEER REVIEWED**
ATMOSPHERIC FATE: According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere(1), formaldehyde, which has a vapor pressure of 3,890 mm Hg at 25 deg C(2), will exist in the gas phase in the ambient atmosphere(SRC). Gas-phase formaldehyde is degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals(SRC); the half-life for this reaction in air is 41 hrs(SRC), calculated from its rate constant of 9.4X10-12 cu cm/molecule-sec at 25 deg C(3). The hydroxy radical initiated oxidation of formaldehyde also occurs in cloud droplets to form formic acid, a component of acid rain(4). Formaldehyde absorbs ultraviolet radiation at wavelengths of >360 nm(5); therefore, formaldehyde may directly photolyze in sunlight(SRC). Formaldehyde has a half-life of 6 hrs in simulated sunlight(5). The predicted half-life of formaldehyde due to photolysis in the lower atmosphere is 1.6 hrs at a solar zenith of 40 degrees(5). Formaldehyde reacts with the NO3 radical by H-atom abstraction with a half-life of 12 days (assuming an average NO3 radical concentration of 2X10+9/cu cm)(6).
[(1) Bidleman TF; Environ Sci Technol 22: 361-367 (1988) (2) Boublik T et al; The vapor pressures of pure substances. Vol. 17. Amsterdam, Netherlands: Elsevier Sci Publ p. 44 (1984) (3) Kwok ESC, Atkinson R; Estimation of hydroxyl radical reaction rate constants for gas-phase organic compounds using a structure-reactivity relationship: an update. Riverside, CA: Univ CA, Statewide Air Pollut Res Ctr. CMA Contract No. ARC-8.0-OR (1994) (4) Chameides WL, Davis DD; Nature 304: 427-9 (1983) (5) Su F et al; J Phys Chem 83: 3185-91 (1979) (4) Calvert JG et al; Science 175: 751-52 (1972) (6) Atkinson R et al; J Phys Chem 88: 1210-5 (1984)]**PEER REVIEWED**
Environmental Biodegradation:
AEROBIC: Formaldehyde, present at 100 mg/l, reached 91% of its theoretical BOD in 2 weeks using an activated sludge inoculum at 30 mg/l and the Japanese MITI test(1). Formaldehyde in aqueous effluent was degraded by activated sludge and sewage in 48-72 hr(2-5). In a die-away test using water from a stagnant lake, degradation was complete in 30 hours under aerobic conditions(2). Other biodegradation screening tests gave half-lives ranging from <1 to 17.3 days(6-11).
[(1) CITI; Biodegradation and Bioaccumulation Data of Existing Chemicals. Formaldehyde (50-00-0). Available from the Database Query page at http://www.cerij.or.jp/ceri_en/index_e4.shtml as May 8, 2001. (2) Kitchens JF et al; Investigation of selected potential environmental contaminants; formaldehyde. Washington DC: USEPA, Off Tox Subst USEPA 560/2-76-009 p. 99-110 (1976) (3) Hatfield R; Ind Eng Chem 49: 192-6 (1957) (4) Heukelekian H, Rand MC; J Water Pollut Control Assoc 29: 1040-53 (1955) (5) Verschueren K; Handbook of environmental data on organic chemicals 4th ed. NY, NY: John Wiley and Sons, p. 1170-4 (2001) (6) Belly RT, Goodhue CT; pp. 1103-7 in Proc Int Biodegrad Symposium 3rd (1976) (7) Dickerson Bw et al; 9th Industrial Waste Conf Purdue Univ Ext Ser 87: 311 (1955) (8) Gellman I, Heukelekian H; J Water Pollut Contr Assoc 27: 1040-53 (1955) (9) Pauli O, Franke G; pp. 52-60 in Biodeter Mater Proc Int Biodeter Symp 2nd. (1971) (10) Stafford W, Northup HJ; Amer Dyestuff Reporter 44:355-9 (1955) (11) Hatfield R Ind Eng Chem 49: 192-6 (1957)]**PEER REVIEWED**
ANAEROBIC: In a die-away test using water from a stagnant lake, degradation was complete in 48 hours under anaerobic conditions(1).
[(1) Kitchens JF et al; Investigation of selected potential environmental contaminants; formaldehyde. Washington DC: USEPA, Off Tox Subst USEPA 560/2-76-009 p. 99-110 (1976)]**PEER REVIEWED**
Environmental Abiotic Degradation:
The rate constant for the gas-phase reaction of formaldehyde with photochemically-produced hydroxyl radicals is 9.4X10-12 cu cm/molecule-sec at 25 deg C(1). This corresponds to an atmospheric half-life of about 41 hrs at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm(1). The hydroxy radical initiated oxidation of formaldehyde also occurs in cloud droplets to form formic acid, a component of acid rain(2). Formaldehyde has a photolytic half-life of 6 hrs in simulated sunlight(3). There are two photolytic pathways, one producing hydrogen gas and carbon monoxide, and the other producing H and HCO radicals(4,5). The predicted half-life of formaldehyde due to photolysis in the lower atmosphere is 1.6 hrs at a solar zenith of 40 degrees(4). Formaldehyde reacts with NO3 radicals by H-atom abstraction with a half-life of 12 days (assuming an average NO3 radical concn of 2X10+9/cu cm)(6). In water, formaldehyde is hydrated; the hydrate does not have a chromophore that is capable of absorbing sunlight and photolytically decomposing(2). Formaldehyde is not expected to undergo hydrolysis in the environment because of the lack of hydrolyzable functional groups(7). Solutions containing formaldehyde are unstable, both oxidizing slowly to form formic acid and polymerizing to form oligomers(8). In the presence of air and moisture, polymerization readily takes place in concentrated solutions at room temperatures to form paraformaldehyde, a solid mixture of linear polyoxymethylene glycols containing 90-99% formaldehyde(9). In dilute aqueous solution, formaldehyde exists almost exclusively as the hydrated gem-diol (CH2(OH)2)(10).
[(1) Kwok ESC, Atkinson R; Estimation of hydroxyl radical reaction rate constants for gas-phase organic compounds using a structure-reactivity relationship: an update. Riverside, CA: Univ CA, Statewide Air Pollut Res Ctr. CMA Contract No. ARC-8.0-OR (1994) (2) Chameides WL, Davis DD; Nature 304: 427-9 (1983) (3) Su F et al; J Phys Chem 83: 3185-91 (1979) (4) Calvert JG et al; Science 175: 751-52 (1972) (5) Lowe DC et al; Geophys Res Letters 7: 825-8 (1980) (6) Atkinson R et al; J Phys Chem 88: 1210-5 (1984) (7) Lyman WJ et al; Handbook of Chemical Property Estimation Methods. Washington, DC: Amer Chem Soc pp. 7-4, 7-5 (1990) (8) Gerberich HR, Seaman GC; Kirk-Othmer Encycl Chem Technol. 4th ed. NY, NY: John Wiley and Sons 11: 931 (1994) (9) USEPA; Locating and Estimating Air Emissions From Sources of Formaldehyde. USEPA-450/4-84-007E (1984) (10) Dong S, Dasgupta PK; Environ Sci Technol 20: 637-40 (1986)]**PEER REVIEWED**
Environmental Bioconcentration:
Experiments performed on a variety of fish and shrimp show no bioconcentration of formaldehyde(1,2).
[(1) Hose JE, Lightner DV; Aquaculture 21: 197-201 (1980) (2) Sills JB, Allen JL; Prog Fish Cult 4: 67-8 (1979)]**PEER REVIEWED**
Soil Adsorption/Mobility:
The Koc of formaldehyde is estimated as 37(SRC), using a log Kow of 0.35(1) and a regression-derived equation(2). According to a classification scheme(3), this estimated Koc value suggests that formaldehyde is expected to have very high mobility in soil(SRC). Formaldehyde gas adsorbs on clay minerals to a degree at high gas concns which is an important quality in its use as a soil fumigant(4).
[(1) Hansch C et al; Exploring QSAR. Hydrophobic, Electronic, and Steric Constants. ACS Prof Ref Book. Heller SR, consult. ed., Washington, DC: Amer Chem Soc p. 3 (1995) (2) Lyman WJ et al; Handbook of Chemical Property Estimation Methods. Washington, DC: Amer Chem Soc pp. 4-9 (1990) (3) Swann RL et al; Res Rev 85: 17-28 (1983) (4) De SK, Chandra K; Sci Cult 44: 462-4 (1978)]**PEER REVIEWED**
Volatilization from Water/Soil:
The Henry's Law constant for formaldehyde is 3.4X10-7 atm-cu m/mole(1). This Henry's Law constant indicates that formaldehyde is expected to be essentially nonvolatile from water surfaces(2). The volatilization of formaldehyde from dry soil surfaces occurs because it is a gas under ambient conditions(3).
[(1) Betterton EA, Hoffmann MR; Environ Sci Technol 22: 1415-8 (1988) (2) Lyman WJ et al; Handbook of Chemical Property Estimation Methods. Washington, DC: Amer Chem Soc pp. 15-1 to 15-29 (1990) (3) Boublik T et al; The vapor pressures of pure substances. Vol. 17. Amsterdam, Netherlands: Elsevier Sci. Publ p. 44 (1984)]**PEER REVIEWED**
Environmental Water Concentrations:
DRINKING WATER: Formaldehyde was not detected in National Organics Reconnaissance Survey of Suspected Carcinogens in Drinking Water(1).
[(1) USEPA; Preliminary Assessment of Suspected Carcinogens in Drinking Water. Office of Toxic Substances (1975)]**PEER REVIEWED**
SURFACE WATER: Formaldehyde was detected at 14 heavily industrialized river basins in the US; 1 of 204 sites were positive at a concn of 12 ppb(1). Formaldehyde was detected only in hypolimnion of stagnant lake in Japan(2).
[(1) Ewing BB et al; Monitoring to Detect Previously Unrecognized Pollutants in Surface Waters. USEPA 560/6-77-015, appendix USEPA 560/6-77-015a p. 75 (1977) (2) Kitchens JF et al; Investigation of Selected Potential Environmental Contaminants: Formaldehyde. Washington DC: USEPA, Off Tox Subst USEPA 560/2-76-009 p. 92-110 (1976)]**PEER REVIEWED**
SEAWATER: Formaldehyde was not detected in surface waters(1).
[(1) Kitchens JF et al; Investigation of Selected Potential Environmental Contaminants: Formaldehyde. Washington DC: USEPA, Off Tox Subst USEPA 560/2-76-009 p. 92-110 (1976)]**PEER REVIEWED**
RAIN/FOG: Formaldehyde levels in rainwater collected in California were low, ranging from not detected to 0.06 ug/ml(1). The concn of formaldehyde in rainwater from Mainz and Deuselbach, Germany, and Ireland ranged from 0.111 to 0.174 ppm(2). Formaldehyde was detected in rain water collected from Enewetek Atoll in the Central Pacific Ocean at range of concns from 6.2-11.3 ppb(3). Concns of free formaldehyde measured in fogwater in Corvallis, OR, ranged from 0.4 to 3 mg/l with a volume-weighted mean of 1.8 mg/l(4). Free formaldehyde concns in fogwater in Riverside, CA, ranged from 0.12 to 6.8 mg/l, with approximately half of the samples less than 3 mg/l(5) Status cloud water at Henninger Flats, CA, which is typically is highly acidic and concentrated in inorganic pollutants, had concns of free formaldehyde ranging from 1.4 to 1.8 mg/l, comparable to mid-range Corvallis fogwater concns(5). Formaldehyde concns ranging from 0.3 to 4.3 mg/l were found in cloud water samples collected in the Los Angeles Basin(6). Formaldehyde concns in mist samples in Long Beach and Marina del Ray, CA, were 0.25 and 0.56 mg/l, respectively(1). The concn of formaldehyde in ice fog from Fairbanks, AK ranged from 0.50 to 1.16 ppm(1). The mean (arithmetic) concn of formaldehyde in rain water fractions from Mexico City and Rancho Viejo, Mexico ranged from 0.13 to 0.42 mg/l and from 0.18 to 0.21 mg/l, respectively(7). The mean values of formaldehyde in rain water at 3 locations in Kobe City (Japan) from Jan 1992 to Dec 1992 were 0.032 mg/l (range, 0.001-0.15 mg/l), 0.035 mg/l (range, 0.001-0.18 mg/l), and 0.016 mg/l (range, 0.001-0.04 mg/l), respectively(8).
[(1) Grosjean D, Wright B; Atmos Environ 17: 2093-6 (1983) (2) Klippel W, Warneck P; Geophys Res Lett 5: 177-9 (1978) (3) Zafiriou OC et al; Geophys Res Lett 7: 341-4 (1980) (4) Muir PS; J Air Waste Manage 41: 32-8 (1991) (5) Igawa M et al; Environ Sci Technol 23: 556-61 (1989) (6) Richards LW et al; Atmos Environ 17: 911-4 (1983) (7) Baez AP et al; Environ Pollut 79: 271-5 (1993) (8) Adachi A, Kobayashi T; Bull Environ Contam Toxicol 57: 556-9 (1996)]**PEER REVIEWED**
Effluent Concentrations:
The major contributors to indoor formaldehyde are pressed wood products and foam insulation containing urea-formaldehyde resins(1). Common indoor combustion sources include gas burners and ovens, kerosene heaters, and cigarettes(2). The emissions from cigarette smoking are 2,000 ug/cigarette(3). Formaldehyde was detected in 3 effluent streams, two from chemical plants and one from a sewage treatment plant(4). Effluent from urea and melamine production contained 4% formaldehyde and from phenolic resin production contained 0.1% formaldehyde(5). Emissions from a wastewater treatment plant in Los Angeles, CA (Hyperion) was 391 kg/yr(6).
[(1) ATSDR; Toxicological Profile for Formaldehyde. Atlanta, GA: ATSDR, Contract No. 205-93-0606 p. 283 (1998) (2) Matthews TC et al; in Indoor Air and Human Health. Inc, Gammage RB, Kaye SV, eds. Chelsea, MI: Lewis Pub (1985) (3) Verschueren K; Handbook of Environmental Data of Organic Chemicals. 4th ed. NY, NY: John Wiley and Sons Inc 1: 1170-4 (2001) (4) Shakelford WM, Keith LH; Frequency of Organic Compounds Identified in Water. p.136 USEPA 600/4-76-062 (1976) (5) IARC; Monograph in Some Industrial Chemicals and Dyestuffs 29: 345-89 (1982) (6) Mayer GJ et al; Water Environ Res 66: 140-4 (1994)]**PEER REVIEWED**
Formaldehyde is released to outdoor air from both natural and industrial sources; combustion processes account directly or indirectly for most of the formaldehyde entering the atmosphere(1). Before 1975, automobiles were found to emit about 2.8X10+8 kg of formaldehyde each year(2); emissions have been reduced since the introduction of the catalytic converter in 1975(3). The concn of formaldehyde in diesel exhaust was 18 ppm(4). The concn of formaldehyde in the exhaust of a 1970 Ford Maverick gasoline engine ranged from 11 to 15 ppm(4). Formaldehyde concns in jet engine exhaust have been found to range from 0.761 to 1.14 ppm(5). Formaldehyde was released from 1982 consumer products, e.g., pressed wood products (range, 0.4-21 ug/g product per day); new clothes not previously washed (range, 0.2-4.9 ug/g product per day); fiberglass insulation products (range, 0.03-2.3 ug/g product per day); paper plates and cups (range, 0.03-0.36 ug/g product per day), fabrics (range, 0.01-3 ug/g product per day), and carpets (range, not detected-0.06 ug/g product per day)(4).
[(1) ATSDR; Toxicological Profile for Formaldehyde. Atlanta, GA: ATSDR, Contract No. 205-93-0606 p. 283 (1998) (2) Kitchens JF et al; Investigations of selected potential environmental contaminants: Formaldehyde. Washington DC: USEPA, Off Tox Subst USEPA 560/2-76-009 (1976) (3) Zweidinger RB at al; Environ Sci Technol 22: 956-62 (1988) (4) Verschueren K; Handbook of Environmental Data of Organic Chemicals. 4th ed. NY, NY: John Wiley and Sons Inc, 1: 1170-4 (2001) (5) Miyamoto Y; Aviation, Space, and Environmental Medicine 57: 1104-8 (1986)]**PEER REVIEWED**
Atmospheric Concentrations:
RURAL/REMOTE: Ambient levels of formaldehyde are generally <1 ug/cu m in remote areas; for example, in the unpopulated Eniwetok Atoll in the Pacific Ocean, a mean of 0.5 ug/cu m and a max of 1.0 ug/cu m formaldehyde were measured in outdoor air(1). The avg and range of concns of formaldehyde in clean marine air are generally <0.5 ppb and <0.03 to 4 ppb, respectively(2-7).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 242 (1995) (2) Platt V, Perner D; J Geophys Res 85: 7453-8 (1980) (3) Zafiriou OC et al; Geophys Res Lett 7: 341-4 (1980) (4) Fushimi K, Miyake Y; J Geophys Res 85: 7533-6 (1980) (5) Neitzert V, Seiler W; Geophys Res Lett 8: 79-82 (1981) (6) Lowe DC et al; Environ Sci Technol 15: 819-23 (1981) (7) Platt U et al; J Geophys Res 84: 6329-35 (1979)]**PEER REVIEWED**
URBAN/SUBURBAN: Outdoor air concns in urban environments are more variable and depend on local conditions(1). They are usually 1-20 ug/cu m(1). A major source of formaldehyde in urban air is incomplete combustion of hydrocarbon fuels; urban air concn in heavy traffic or during severe inversions can range up to 100 ug/cu m(1). The concn of formaldehyde was measured at various sites in US(7); 26% of 749 samples were positive(2); 25% of samples had concns >2.7 ppb with a max concn of 27 ppb(2). Six cities in US had avg concns of formaldehyde ranging from 11.3 to 20.6 ppb with a max concn of 4 ppb(3,4). The daily mean and 1 hr max concn of formaldehyde at 4 cities in New Jersey ranged from 3.8 to 6.6 ppb and 14 to 20 ppb, respectively(5). Two cities in Southern California had concns of formaldehyde ranging from 2 to 48 ppb during photochemical smog episodes(6). From Aug 1979 to Aug 1980, the mean and range of concns of formaldehyde were 1.28 ppb and 0.11 to 10 ppb (N=174), respectively, at a moderately polluted area near Julich, Germany(7). The concn of formaldehyde decreases as one goes up several hundred feet in altitude(8).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 242 (1995) (2) Brodzinsky R, Singh HB; Volatile Organic Chemicals in the Atmosphere: An Assessment of Available Data p. 119 SRI 68-02-3452 (1982) (3) Singh HB et al; Atmospheric Measurements of Selected Hazards Organic Chemicals USEPA 600/13-80-072 (1981) (4) Singh HB et al; Environ Sci Technol 16: 872-80 (1982) (5) Cleveland WS et al; Atmos Environ 11: 357-60 (1977) (6) Grosjean D; Environ Sci Technol 16: 254-62 (1982) (7) Lowe DC, Schmidt U; J Geophys Res 88: 10,844-58 (1983) (8) Lowe DC et al; Geophys Res Lett 7: 825-8 (1980)]**PEER REVIEWED**
URBAN/SUBURBAN: The concn of formaldehyde at the Univ of Mexico campus between Mar-May 1993 ranged from 9.9 to 110.4 ppb(1). Formaldehyde was detected in air from San Paulo, Brazil (range, 8.5-19.3 ppb; July 1988)(2), Athens, Greece (range, 15-25 ppb in Winter 1991; range, 6-20 ppb in Spring 1991)(3), and Grenoble, France (range, 2-18 ppb; May 1995)(4). The concn of formaldehyde in air from Rome, Italy ranged from 8.8-27.7 ppb between Jun-Jul 1994 and from 8.2-17.6 ppb between Jan-Mar 1995(5). Ambient levels of formaldehyde from 24 samples were collected every day at 6 Southern California locations between 9/2/88 and 9/25/89; avg concns in Anaheim, Azusa, Burbank, Hawthorne, Upland, and W. Los Angeles, CA were 5.3 ppb (max, 25.3 ppb), 5.0 ppb (max, 20.7 ppb), 6.0 ppb (max, 26.0 ppb), 6.0 ppb (max, 29.4 ppb), 5.3 ppb (max, 29.2 ppb), and 6.1 ppb (max, 25.8 ppb), respectively(6). The avg concn of formaldehyde in ambient air from Columbus, OH (June-July 1989) was 3.8 ug/cu m(7).
[(1) Baez AP et al; Environ Pollut 89: 163-7 (1995) (2) Grosjean D et al; Atmos Environ 24B: 101-6 (1990) (3) Viras LG et al; Fres Environ Bull 1: 73-8 (1992) (4) Ferrari CP et al; Chemosphere 37: 1587-1601 (1998) (5) Possanzini M et al; Atmos Environ 30: 3757-64 (1996) (6) Grosjean D; Environ Sci Technol 25: 710-15 (1991) (7) Mukund R et al; Atmos Environ 30: 3457-70 (1996)]**PEER REVIEWED**
INDOOR AIR: The levels of formaldehyde in indoor air are often higher than those outside(1). The concn in dwellings depend on the sources of formaldehyde that are present, the age of the source materials, ventilation, temperature, and humidity(1). Major sources of formaldehyde in some dwellings have been reported to be off-gassing of urea-formaldehyde foam insulation and particle board(1). The mean level in conventional homes with no urea-formaldehyde foam insulation were 25-60 ug/cu m(1). Studies conducted in Denmark, Sweden, Germany, and the US frequently found indoor formaldehyde levels in excess of 0.12 ppm and in several cases >3.0 ppm(2). In an energy efficient research house, formaldehyde levels were 65 ppb without furniture, 182 ppb with furniture, 212 ppb occupied during day, 114 ppb occupied during night(2).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 242 (1995) (2) Council on Environmental Quality; Environmental Quality-1980. p. 185-7 (1980)]**PEER REVIEWED**
INDOOR AIR: Many studies have been reported since the late 1970s of formaldehyde levels in mobile homes (caravans)(1). In 3 states, mobile homes (with a filed complaint) had mean levels of formaldehyde ranging from 0.1 to 0.88 ppm and up to 3.0 ppm(2). Non-complaint mobile homes in Wisconsin which were <3 yrs old had a mean concn of formaldehyde of 0.54 ppm; mobile homes >3 yr old had a mean concn of 0.19 ppm(3). Homes in Houston, TX were found to have concns >0.10 ppm in 19% of those tested(4). The levels of formaldehyde appear to decrease as the mobile home (and its formaldehyde-based resins) age, with a half-life of 4 to 5 years(1). In the early 1980s, a mean level of 0.4 ppm and individual measurements as high as several ppm were measured in new mobile homes. As a result of new standards set in the mid-1980s for building materials used in mobile homes and voluntary reductions by the manufacturers, formaldehyde levels in mobile homes are now typically around 0.1 ppm or less(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 242 (1995) (2) National Research Council; Formaldehyde and Other Aldehydes p. 5-13 USEPA 600/6-82-002 (1982) (3) Lowe DC, Schmidt U; J Geophys Res 88: 10,844-58 (1983) (4) Stack TH; J Air Pollut Control Assoc 37: 913-8 (1987)]**PEER REVIEWED**
Food Survey Values:
Formaldehyde occurs naturally in foods, and foods may be contaminated as a result of fumigation (of e.g. grain), cooking (as a combustion product) and release from formaldehyde resin-based tableware(1). It has been used as a bacteriostatic agent in some foods, such as cheese(1). Fruits and vegetables typically contain 3-60 mg/kg, and meat and fish, 6-20 mg/kg(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 243 (1995)]**PEER REVIEWED**
Fish/Seafood Concentrations:
Shellfish typically contain 1-100 mg/kg of formaldehyde(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 243 (1995)]**PEER REVIEWED**
Milk Concentrations:
Milk and milk products typically contain about 1 mg/kg of formaldehyde(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 62: 243 (1995)]**PEER REVIEWED**
Other Environmental Concentrations:
LEVEL IN SCHOOL BUILDING WAS 0.3 TO 0.9 PPM DURING SUMMER. SOURCE OF EMISSION WAS UREA-FORMALDEHYDE RESIN BINDER USED IN FURNITURE & FLOOR COVERING. AFTER 1 YR, NO FORMALDEHYDE VAPOR WAS RELEASED.
[DEIMEL M; ORG VERUNREINIG UMWELT: ERKENNEN, BEWERTEN, VERMINDERN, (TAG): 416-27 (1978)]**PEER REVIEWED**
PAPER PLATES & CUPS, LADIES DRESSES, MEN'S SHIRTS, 100% COTTON DRAPERY FABRIC, GIRLS DRESSES (POLYESTER/COTTON), LATEX-BACKED FABRIC, FOAM-BACKED CARPET & CHILD'S CLOTHES (65% POLYESTER/35% COTTON) ARE ONLY A FEW OF THE 39 SAMPLE TYPES STUDIED WHICH RELEASED FORMALDEHYDE AT RATE OF 1 TO 34,000 UG/SQ M/DAY.
[PICKRELL JA ET AL; INHALATION TOXICOLOGY RESEARCH INSTITUTE, LOVELACE BIOMEDICAL & ENVIRONMENTAL RESEARCH INSTITUTE, PO BOX 5890, ALBUQUERQUE, NM 87185 (FEBRUARY 1982)]**PEER REVIEWED**
Free formaldehyde is emitted from formaldehyde resins used in durable-press cotton when they are heat-cured and stored; in the US, the concn in 112 fabric samples ranged from 1 to 3517 mg/kg; 18 samples had a free formaldehyde content greater than 750 mg/kg(1).
[(1) IARC; Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva, Switzerland: WHO 29: 358 (1982)]**PEER REVIEWED**
Environmental Standards & Regulations:
FIFRA Requirements:
Formaldehyde not more than 1% of pesticide formulation is exempted from the requirement of a tolerance when used as a preservative for formulation in accordance with good agricultural practice as inert (or occasionally active) ingredients in pesticide formulations applied to growing crops only.
[40 CFR 180.1001(d) (7/1/2000]**PEER REVIEWED**
As the federal pesticide law FIFRA directs, EPA is conducting a comprehensive review of older pesticides to consider their health and environmental effects and make decisions about their future use. Under this pesticide reregistration program, EPA examines health and safety data for pesticide active ingredients initially registered before November 1, 1984, and determines whether they are eligible for reregistration. In addition, all pesticides must meet the new safety standard of the Food Quality Protection Act of 1996. Formaldehyde is found on List A, which contains most food use pesticides and consists of the 194 chemical cases (or 350 individual active ingredients) for which EPA issued registration standards prior to FIFRA, as amended in 1988. Case No: 0556; Pesticide type: fungicide, antimicrobial; Registration Standard Date: 05/31/88; Case Status: OPP is reviewing data from the pesticide's producers regarding its human health and/or environmental effects, or OPP is determining the pesticide's eligibility for reregistration and developing the Reregistration Eligibility Decision (RED) document.; Active ingredient (AI): Formaldehyde; AI Status: The producers of the pesticide has made commitments to conduct the studies and pay the fees required for reregistration, and are meeting those commitments in a timely manner.
[USEPA/OPP; Status of Pesticides in Registration, Reregistration and Special Review p.122 (Spring, 1998) EPA 738-R-98-002]**PEER REVIEWED**
CERCLA Reportable Quantities:
Persons in charge of vessels or facilities are required to notify the National Response Center (NRC) immediately, when there is a release of this designated hazardous substance, in an amount equal to or greater than its reportable quantity of 100 lb or 45.4 kg. The toll free number of the NRC is (800) 424-8802; In the Washington D.C. metropolitan area (202) 426-2675. The rule for determining when notification is required is stated in 40 CFR 302.4 (section IV. D.3.b).
[40 CFR 302.4 (7/1/2000]**PEER REVIEWED**
Releases of CERCLA hazardous substances are subject to the release reporting requirement of CERCLA section 103, codified at 40 CFR part 302, in addition to the requirements of 40 CFR part 355. Formaldehyde is an extremely hazardous substance (EHS) subject to reporting requirements when stored in amounts in excess of its threshold planning quantity (TPQ) of 500 lbs.
[40 CFR 355 (7/1/2000]**PEER REVIEWED**
RCRA Requirements:
U122; As stipulated in 40 CFR 261.33, when formaldehyde, as a commercial chemical product or manufacturing chemical intermediate or an off-specification commercial chemical product or a manufacturing chemical intermediate, becomes a waste, it must be managed according to Federal and/or State hazardous waste regulations. Also defined as a hazardous waste is any residue, contaminated soil, water, or other debris resulting from the cleanup of a spill, into water or on dry land, of this waste. Generators of small quantities of this waste may qualify for partial exclusion from hazardous waste regulations (40 CFR 261.5).
[40 CFR 261.33 (7/1/2000]**PEER REVIEWED**
Atmospheric Standards:
This action promulgates standards of performance for equipment leaks of Volatile Organic Compounds (VOC) in the Synthetic Organic Chemical Manufacturing Industry (SOCMI). The intended effect of these standards is to require all newly constructed, modified, and reconstructed SOCMI process units to use the best demonstrated system of continuous emission reduction for equipment leaks of VOC, considering costs, non air quality health and environmental impact and energy requirements. Formaldehyde is produced, as an intermediate or a final product, by process units covered under this subpart.
[40 CFR 60.489 (7/1/2000]**PEER REVIEWED**
Listed as a hazardous air pollutant (HAP) generally known or suspected to cause serious health problems. The Clean Air Act, as amended in 1990, directs EPA to set standards requiring major sources to sharply reduce routine emissions of toxic pollutants. EPA is required to establish and phase in specific performance based standards for all air emission sources that emit one or more of the listed pollutants. Formaldehyde is included on this list.
[Clean Air Act as amended in 1990, Sect. 112 (b) (1) Public Law 101-549 Nov. 15, 1990]**PEER REVIEWED**
Clean Water Act Requirements:
Formaldehyde is designated as a hazardous substance under section 311(b)(2)(A) of the Federal Water Pollution Control Act and further regulated by the Clean Water Act Amendments of 1977 and 1978. These regulations apply to discharges of this substance. This designation includes any isomers and hydrates, as well as any solutions and mixtures containing this substance.
[40 CFR 116.4 (7/1/2000]**QC REVIEWED**
Federal Drinking Water Guidelines:
EPA 1000 ug/l
[USEPA/Office of Water; Federal-State Toxicology and Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines (11/93), p. ]**QC REVIEWED**
State Drinking Water Guidelines:
(CA) CALIFORNIA 100 ug/l
[USEPA/Office of Water; Federal-State Toxicology and Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines (11/93), p. ]**QC REVIEWED**
(FL) FLORIDA 600 ug/l
[USEPA/Office of Water; Federal-State Toxicology and Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines (11/93), p. ]**QC REVIEWED**
(ME) MAINE 140 ug/l
[USEPA/Office of Water; Federal-State Toxicology and Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines (11/93), p. ]**QC REVIEWED**
(MN) MINNESOTA 1000 ug/l
[USEPA/Office of Water; Federal-State Toxicology and Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines (11/93), p. ]**QC REVIEWED**
(NJ) NEW JERSEY 100 ug/l
[USEPA/Office of Water; Federal-State Toxicology and Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines (11/93), p. ]**QC REVIEWED**
(WI) WISCONSIN 1000 ug/l
[USEPA/Office of Water; Federal-State Toxicology and Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines (11/93), p. ]**QC REVIEWED**
FDA Requirements:
Formaldehyde is an indirect food additive for use only as a component of adhesives.
[21 CFR 175.105 (4/1/2000]**PEER REVIEWED**
Formaldehyde is a food additive permitted in feed and drinking water of animals.
[21 CFR 573.460 (4/1/2000]**PEER REVIEWED**
Allowable Tolerances:
Formaldehyde not more than 1% of pesticide formulation is exempted from the requirement of a tolerance when used as a preservative for formulation in accordance with good agricultural practice as inert (or occasionally active) ingredients in pesticide formulations applied to growing crops only.
[40 CFR 180.1001(d) (7/1/2000]**PEER REVIEWED**
Chemical/Physical Properties:
Molecular Formula:
C-H2-O
**PEER REVIEWED**
Molecular Weight:
30.03
[Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 717]**PEER REVIEWED**
Color/Form:
Clear, water-white, very slightly acid, gas or liquid.
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
Formaldehyde solution is a clear, colorless or nearly colorless liquid ...
[Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1110]**PEER REVIEWED**
Nearly colorless gas [Note: Often used in an aqueous solution].
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
Odor:
Pungent, suffocating odor.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
Boiling Point:
-19.5 deg C
[Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 717]**PEER REVIEWED**
Melting Point:
-92 deg C
[Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 717]**PEER REVIEWED**
Corrosivity:
Aqueous formaldehyde is corrosive to carbon steel, but formaldehyde in vapor phase is not.
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 239 (1980)]**PEER REVIEWED**
Critical Temperature & Pressure:
Critical temperature: 137.2-141.2 deg C; critical pressure: 6.784-6.637 MPa (to convert MPa to atm, divide by 0.101)
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 231 (1980)]**PEER REVIEWED**
Density/Specific Gravity:
1.067 (Air= 1)
[Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 717]**PEER REVIEWED**
Dissociation Constants:
pKa = 13.27 @ 25 deg C
[Serjeant, E.P., Dempsey B.; Ionisation Constants of Organic Acids in Aqueous Solution. International Union of Pure and Applied Chemistry (IUPAC). IUPAC Chemical Data Series No. 23, 1979. New York, New York: Pergamon Press, Inc., p. 9]**PEER REVIEWED**
Heat of Combustion:
570.7 kJ/mol (gas)
[Lide, DR (ed.). CRC Handbook of Chemistry and Physics. 81st Edition. CRC Press LLC, Boca Raton: FL 2000, p. 5-89]**PEER REVIEWED**
Heat of Vaporization:
5,917.9 gcal/gmole (to convert to J/g mole, multiply by 4.184)
[Weast, R.C. (ed.) Handbook of Chemistry and Physics. 67th ed. Boca Raton, FL: CRC Press, Inc., 1986-87., p. C-671]**PEER REVIEWED**
Octanol/Water Partition Coefficient:
log Kow= 0.35
[Hansch, C., Leo, A., D. Hoekman. Exploring QSAR - Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society., 1995., p. 3]**PEER REVIEWED**
pH:
pH: 2.8 to 4.0 /Formaldehyde soln/
[Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 717]**PEER REVIEWED**
Solubilities:
Soluble in alcohol, ether, acetone
[Lide, DR (ed.). CRC Handbook of Chemistry and Physics. 81st Edition. CRC Press LLC, Boca Raton: FL 2000, p. 3-166]**PEER REVIEWED**
SOL IN BENZENE
[Lide, D.R. (ed). CRC Handbook of Chemistry and Physics. 72nd ed. Boca Raton, FL: CRC Press, 1991-1992., p. 3-248]**PEER REVIEWED**
In water, 4.00X10+5 mg/l @ 20 deg C
[Pickrell JA et al; Environ Sci Technol 17: 753-7 (1983)]**PEER REVIEWED**
Spectral Properties:
MAX ABSORPTION (GAS): 155.5 NM (LOG E= 4.37); 175 NM (LOG E= 4.26)
[Weast, R.C. (ed.). Handbook of Chemistry and Physics. 60th ed. Boca Raton, Florida: CRC Press Inc., 1979., p. C-309]**PEER REVIEWED**
Index of refraction: 1.3746 at 20 deg C/D /Formaldehyde soln/
[Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 717]**PEER REVIEWED**
IR: 2538 (Sadtler Research Laboratories Prism Collection)
[Lide, D.R., G.W.A. Milne (eds.). Handbook of Data on Organic Compounds. Volume I. 3rd ed. CRC Press, Inc. Boca Raton ,FL. 1994., p. V3 2808]**PEER REVIEWED**
UV: 3-1 (Organic Electronic Spectral Data, Phillips et al, John Wiley & Sons, New York)
[Lide, D.R., G.W.A. Milne (eds.). Handbook of Data on Organic Compounds. Volume I. 3rd ed. CRC Press, Inc. Boca Raton ,FL. 1994., p. V3 2808]**PEER REVIEWED**
MS: 37883 (National Institute of Standards and Technology); 74 (Atlas of Mass Spectral Data, John Wiley and Sons, NY)
[Lide, D.R., G.W.A. Milne (eds.). Handbook of Data on Organic Compounds. Volume I. 3rd ed. CRC Press, Inc. Boca Raton ,FL. 1994., p. V3 2808]**PEER REVIEWED**
Vapor Pressure:
3,890 mm Hg @ 25 deg C
[Boublik, T., Fried, V., and Hala, E., The Vapour Pressures of Pure Substances. Second Revised Edition. Amsterdam: Elsevier, 1984., p. 44]**PEER REVIEWED**
Other Chemical/Physical Properties:
Freezing point: -117 deg C /Formaldehyde, 37% uninhibited/
[Flick, E.W. (ed.). Industrial Solvents Handbook 4 th ed. Noyes Data Corporation., Park Ridge, NJ., 1991., p. 511]**PEER REVIEWED**
Formaldehyde solution is a clear, colorless or nearly colorless liquid having a pungent, irritating odor. /Formaldehyde soln/
[Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1110]**PEER REVIEWED**
Specified gravity: 0.816 g at 20/20 deg C /Formaldehyde, 37% uninhibited/
[Flick, E.W. (ed.). Industrial Solvents Handbook 4 th ed. Noyes Data Corporation., Park Ridge, NJ., 1991., p. 511]**PEER REVIEWED**
Vapor pressure: 1 mm Hg /Formalin/
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
In the presence of air and moisture, polymerization readily takes place in concentrated solutions at room temperatures to form paraformaldehyde, a solid mixture of linear polyoxymethylene glycols containing 90-99% formaldehyde.
[USEPA; Locating and Estimating Air Emissions From Sources of Formaldehyde. USEPA-450/4-84-007E (1984)]**PEER REVIEWED**
Boiling point: - 19.1 deg C /Formaldehyde 37% uninhibited/
[Flick, E.W. (ed.). Industrial Solvents Handbook 4 th ed. Noyes Data Corporation., Park Ridge, NJ., 1991., p. 511]**PEER REVIEWED**
In the presence of air, formaldehyde is oxidized to formic acid.
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium, 11 th ed., British Crop Protection Council, Surrey, England 1997, p. 622]**PEER REVIEWED**
Henry's Law constant = 3.37X10-7 atm cu m/mol @ 25 deg C
[Betterton EA, Hoffmann MR; Environ Sci Technol 22: 1415-8 (1988)]**PEER REVIEWED**
Hydroxyl radical reaction rate constant = 9.37X10-12 cu cm/molecule-sec @ 25 deg C
[Kwok ESC, Atkinson R; Estimation of hydroxyl radical reaction rate constants for gas-phase organic compounds using a structure-reactivity relationship: an update. Riverside, CA: Univ CA, Statewide Air Pollut Res Ctr. CMA Contract No. ARC-8.0-OR (1994)]**PEER REVIEWED**
Chemical Safety & Handling:
DOT Emergency Guidelines:
Fire or explosion: Flammable/combustible materials. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Those substances desigmnated with a "P" may polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. /Formaldehyde, solution, flammable; Formaldehyde, solutions (Formalin); Formaldehyde, solutions, (Formalin) (corrosive)/
[U.S. Department of Transportation. 2000 Emergency Response Guidebook. RSPA P 5800.8 Edition. Washington, D.C: U.S. Government Printing Office, 2000,p. G-132]**QC REVIEWED**
Health: May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. /Formaldehyde, solution, flammable; Formaldehyde, solutions (Formalin); Formaldehyde, solutions, (Formalin) (corrosive)/
[U.S. Department of Transportation. 2000 Emergency Response Guidebook. RSPA P 5800.8 Edition. Washington, D.C: U.S. Government Printing Office, 2000,p. G-132]**QC REVIEWED**
Public safety: CALL Emergency Response Telephone Number ... . Isolate spill or leak area immediately for at least 50 to 100 meters (160 to 330 feet) in all directions. Keep unauthorized personnel away. Stay upwind. Keep out of low areas. Ventilate closed spaces before entering. /Formaldehyde, solution, flammable; Formaldehyde, solutions (Formalin); Formaldehyde, solutions, (Formalin) (corrosive)/
[U.S. Department of Transportation. 2000 Emergency Response Guidebook. RSPA P 5800.8 Edition. Washington, D.C: U.S. Government Printing Office, 2000,p. G-132]**QC REVIEWED**
Protective clothing: Wear positive pressure self-contained breathing apparatus (SCBA). Wear chemical protective clothing which is specifically recommended by the manufacturer. It may provide little or no thermal protection. Structural firefighters' protective clothing is recommended for fire situations only; it is not effective in spill situations. /Formaldehyde, solution, flammable; Formaldehyde, solutions (Formalin); Formaldehyde, solutions, (Formalin) (corrosive)/
[U.S. Department of Transportation. 2000 Emergency Response Guidebook. RSPA P 5800.8 Edition. Washington, D.C: U.S. Government Printing Office, 2000,p. G-132]**QC REVIEWED**
Evacuation: ... Fire: If tank, rail car or tank truck is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. /Formaldehyde, solution, flammable; Formaldehyde, solutions (Formalin); Formaldehyde, solutions, (Formalin) (corrosive)/
[U.S. Department of Transportation. 2000 Emergency Response Guidebook. RSPA P 5800.8 Edition. Washington, D.C: U.S. Government Printing Office, 2000,p. G-132]**QC REVIEWED**
Fire: Some of these materials may react violently with water. Small fires: Dry chemical, CO2, water spray or alcohol-resistant foam. Large fires: Water spray, fog or alcohol-resistant foam. Move containers from fire area if you can do it without risk. Dike fire control water for later disposal; do not scatter the material. Do not get water inside containers. Fire involving tanks or car/trailer loads: Fight fire from maximum distance or use unmanned hose holders or monitor nozzles. Cool containers with flooding quantities of water until well after fire is out. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. ALWAYS stay away from tanks engulfed in fire. For massive fire, use unmanned hose holders or monitor nozzles; if this is impossible, withdraw from area and let fire burn. /Formaldehyde, solution, flammable; Formaldehyde, solutions (Formalin); Formaldehyde, solutions, (Formalin) (corrosive)/
[U.S. Department of Transportation. 2000 Emergency Response Guidebook. RSPA P 5800.8 Edition. Washington, D.C: U.S. Government Printing Office, 2000,p. G-132]**QC REVIEWED**
Spill or Leak: Fully encapsulating, vapor protective clothing should be worn for spills and leaks with no fire. ELIMINATE all ignition sources (no smoking, flares, sparks or flames in immediate area). All equipment used when handling the product must be grounded. Do not touch or walk through spilled material. Stop leak if you can do it without risk. Prevent entry into waterways, sewers, basements or confined areas. A vapor suppressing foam may be used to reduce vapors. Absorb with earth, sand or other non-combustible material and transfer to containers (except for Hydrazine). Use clean non-sparking tools to collect absorbed material. Large spills: Dike far ahead of liquid spill for later disposal. Water spray may reduce vapor; but may not prevent ignition in closed spaces. /Formaldehyde, solution, flammable; Formaldehyde, solutions (Formalin); Formaldehyde, solutions, (Formalin) (corrosive)/
[U.S. Department of Transportation. 2000 Emergency Response Guidebook. RSPA P 5800.8 Edition. Washington, D.C: U.S. Government Printing Office, 2000,p. G-132]**QC REVIEWED**
First aid: Move victim to fresh air. Call 911 or emergency medical service. Apply artificial respiration if victim is not breathing. Do not use mouth-to-mouth method if victim ingested or inhaled the substance; induce artificial respiration with the aid of a pocket mask equipped with a one-way valve or other proper respiratory medical device. Administer oxygen if breathing is difficult. Remove and isolate contaminated clothing and shoes. In case of contact with substance, immediately flush skin or eyes with running water for at least 20 minutes. Keep victim warm and quiet. Effects of exposure (inhalation, ingestion or skin contact) to substance may be delayed. Ensure that medical personnel are aware of the material(s) involved, and take precautions to protect themselves. /Formaldehyde, solution, flammable; Formaldehyde, solutions (Formalin); Formaldehyde, solutions, (Formalin) (corrosive)/
[U.S. Department of Transportation. 2000 Emergency Response Guidebook. RSPA P 5800.8 Edition. Washington, D.C: U.S. Government Printing Office, 2000,p. G-132]**QC REVIEWED**
Odor Threshold:
0.5 to 1.0 ppm
[Environment Canada; Tech Info for Problem Spills: Formaldehyde p.1 (1985)]**PEER REVIEWED**
Detection: media= water: 4.99x10+1 ppm /Chemically pure/
[Fazzalari, F.A. (ed.). Compilation of Odor and Taste Threshold Values Data. ASTM Data Series DS 48A (Committee E-18). Philadelphia, PA: American Society for Testing and Materials, 1978., p. 95]**PEER REVIEWED**
Detection: media= water: 2.50x10+1 ppm /Purity not specified/
[Fazzalari, F.A. (ed.). Compilation of Odor and Taste Threshold Values Data. ASTM Data Series DS 48A (Committee E-18). Philadelphia, PA: American Society for Testing and Materials, 1978., p. 95]**PEER REVIEWED**
Recognition: media= air: 1.00 ppm /Chemically pure/
[Fazzalari, F.A. (ed.). Compilation of Odor and Taste Threshold Values Data. ASTM Data Series DS 48A (Committee E-18). Philadelphia, PA: American Society for Testing and Materials, 1978., p. 95]**PEER REVIEWED**
Odor low: 1.4700 mg/cu m; Odor high: 73.5000 mg/cu m
[Ruth JH; Am Ind Hyg Assoc J 47: A-142-51 (1986)]**PEER REVIEWED**
Skin, Eye and Respiratory Irritations:
Contact with the skin causes irritation, tanning effect, and allergic sensitization. Contact with eyes causes irritation, itching, & lacrimation. ...
[Environment Canada; Tech Info for Problem Spills: Formaldehyde p.2 (1985)]**PEER REVIEWED**
Formaldehyde vapor is very irritating to the mucous membranes and toxic to animals, including man.
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 525]**PEER REVIEWED**
Fire Potential:
Flammable
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 524]**PEER REVIEWED**
Flammable liquid when exposed to heat or flame; can react vigorously with oxidizers. ... The gas is a more dangerous fire hazard than the vapor.
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
NFPA Hazard Classification:
Health: 3. 3= Materials that, on short exposure, could cause serious temporary or residual injury, including those requiring protection from all bodily contact. Fire fighters may enter the area only if they are protected from all contact with the material. Full protective clothing, including self-contained breathing apparatus, coat, pants, gloves, boots, and bands around legs, arms, and waist, should be provided. No skin surface should be exposed. /Formaldehyde gas/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Flammability: 4. 4= This degree includes flammable gases, pyrophoric liquids, and Class IA flammable liquids. The preferred method of fire attack is to stop the flow of material or to protect exposures while allowing the fire to burn itself out. /Formaldehyde gas/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Reactivity: 0. 0= This degree includes materials that are normally stable, even under fire exposure conditions, and that do not react with water. Normal fire fighting procedures may be used. /Formaldehyde gas/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Health: 3. 3= Materials that, on short exposure, could cause serious temporary or residual injury, including those requiring protection from all bodily contact. Fire fighters may enter the area only if they are protected from all contact with the material. Full protective clothing, including self-contained breathing apparatus, coat, pants, gloves, boots, and bands around legs, arms, and waist, should be provided. No skin surface should be exposed. /Formaldehyde 37% methanol-free/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Flammability: 2. 2= This degree includes materials that must be moderately heated before ignition will occur and includes Class II and IIIA combustible liquids and solids and semi-solids that readily give off ignitible vapors. Water spray may be used to extinguish fires in these materials because the materials can be cooled below their flash points. /Formaldehyde 37% methanol-free/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Reactivity: 0. 0= This degree includes materials that are normally stable, even under fire exposure conditions, and that do not react with water. Normal fire fighting procedures may be used. /Formaldehyde 37% methanol-free/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Health: 3. 3= Materials that, on short exposure, could cause serious temporary or residual injury, including those requiring protection from all bodily contact. Fire fighters may enter the area only if they are protected from all contact with the material. Full protective clothing, including self-contained breathing apparatus, coat, pants, gloves, boots, and bands around legs, arms, and waist, should be provided. No skin surface should be exposed. /Formaldehyde 37%, 15% methanol/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Flammability: 2. 2= This degree includes materials that must be moderately heated before ignition will occur and includes Class II and IIIA combustible liquids and solids and semi-solids that readily give off ignitible vapors. Water spray may be used to extinguish fires in these materials because the materials can be cooled below their flash points. /Formaldehyde 37%, 15% methanol/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Reactivity: 0. 0= This degree includes materials that are normally stable, even under fire exposure conditions, and that do not react with water. Normal fire fighting procedures may be used. /Formaldehyde 37%, 15% methanol/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Flammable Limits:
Lower flammable limit: 7.0% by volume; Upper flammable limit: 73% by volume /Formaldehyde gas/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Flash Point:
83 deg C, closed cup, 37% aqueous soln- methanol free; 50 deg C, closed cup, aqueous soln with 15% methanol
[American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991., p. 664]**PEER REVIEWED**
Autoignition Temperature:
795 DEG F; (424 DEG C) /FORMALDEHYDE GAS/
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-54]**PEER REVIEWED**
Fire Fighting Procedures:
Use water spray, dry chemical, alcohol foam, or carbon dioxide. Use water to keep fire exposed containers cool. If leak or spill has not ignited, use water spray to disperse vapors, and to protect men attempting to stop leak. Water spray may be used to flush spills away from exposures and to dilute spills to nonflammable mixtures.
[Prager, J.C. Environmental Contaminant Reference Databook Volume 1. New York, NY: Van Nostrand Reinhold, 1995., p. 706]**PEER REVIEWED**
Approach fire from upwind to avoid hazardous vapors and toxic decomposition products. Use water spray, dry chemical, "alcohol resistant" foam, or carbon dioxide.
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 49-72]**PEER REVIEWED**
If material is on fire or involved in a fire: Do not extinguish fire unless flow can be stopped. Use water in flooding quantities as fog. Solid streams of water may be ineffective. Cool all affected containers with flooding quantities of water. Apply water from as far a distance as possible. Use "alcohol" foam, dry chemical or carbon dioxide.
[Association of American Railroads. Emergency Handling of Hazardous Materials in Surface Transportation. Washington, DC: Association of American Railroads, Bureau of Explosives, 1994., p. 516]**PEER REVIEWED**
To fight fire, stop flow of gas (for pure form); alcohol foam for 37% methanol-free form.
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
Firefighting Hazards:
Solutions of formaldehyde in water are considered combustible as the flammable vapors escape and form explosive mixtures with air over a wide range.
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 49-72]**PEER REVIEWED**
Explosive Limits & Potential:
EXPLOSIVE LIMITS: LOWER 7.0%; UPPER 73.0%
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
A moderate explosion hazard when exposed to heat or flame. ... When aqueous formaldehyde solutions are heated above their flash points, a potential for an explosion hazard exists.
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
Hazardous Reactivities & Incompatibilities:
(Amines) exothermic reaction, (AZO cmpd) exothermic reaction giving off nitrogen gas, (caustics) heat generation and violent polymerization, (dithiocarbamates) formation of flammable gasses and toxic fumes, formation of carbon disulfide may result, (alkali and alkaline earth metals) heat generation and formation of flammable hydrogen gas, (nitrides) heat generation, formation of flammable ammonia gas and violent polymerization, (nitro compd) heat generation, (unsaturated aliphatics and sulfides) heat generation, (organic peroxides) violent reaction, (oxidizing agents) heat generation, fire, and decomposition, (reducing agents) heat generation and formation of flammable gasses. /From table/
[USEPA/ORD; A Method for Determining the Compatibility of Haz Wastes (1980) EPA-600/2-80-076 as cited in Environment Canada; Tech Info for Problem Spills: Formaldehyde p.84-87 (1985)]**PEER REVIEWED**
Interaction of nitromethane & formaldehyde in presence of alkali gives ... 2-nitroethanol, ... di- & tri-condensation products. After removal of 2-nitroethanol by vacuum distillation, the residue must be cooled before admitting air into the system to prevent flash explosion or violent fume-off.
[Bretherick, L. Handbook of Reactive Chemical Hazards. 4th ed. Boston, MA: Butterworth-Heinemann Ltd., 1990, p. 164]**PEER REVIEWED**
Formaldehyde ... react violently with 90% performic acid.
[Bretherick, L. Handbook of Reactive Chemical Hazards. 4th ed. Boston, MA: Butterworth-Heinemann Ltd., 1990, p. 153]**PEER REVIEWED**
Formaldehyde and a number of carbonyl cmpd bearing electronegative substituents in the alpha position add to isocyanic acid at temp of -70 deg to 0 deg C to form alpha-hydroxy isocyanates. At higher temp, these isocyanates polymerize and sometimes do so with explosive violence.
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V13 800 (1981)]**PEER REVIEWED**
Reactions with peroxide, nitrogen dioxide, and performic acid, cause explosions.
[Environment Canada; Tech Info for Problem Spills: Formaldehyde p.84-7 (1985)]**PEER REVIEWED**
With magnesium carbonate, explosion is due to the pressure of carbon dioxide formed.
[Environment Canada; Tech Info for Probem Spills: Formaldehyde p.84-7 (1985)]**PEER REVIEWED**
Strong oxidizers, alkalis & acids; phenols; urea [Note: Pure formaldehyde has a tendency to polymerize. Reacts with HCl to form bis-chloromethyl ether].
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
Highly chemically reactive. ... Sensitive to light. Powerful reducing agent.
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 524]**PEER REVIEWED**
Reacts with sodium hydroxide to yield formic acid and hydrogen. Reacts with /nitrogen oxides/ at about 180 deg; the reaction becomes explosive.
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
Hazardous Decomposition:
Uncatalyzed decomposition is very slow below 300 deg C; extrapolation of kinetic data to 400 deg C indicates that the rate of decomposition is about 0.44%/min at 101 kPa (1 atm). The main products are carbon monoxide and hydrogen. Metals such as platinium, copper, chromia, and alumina also catalyze the formation of methanol, methylformate, formic acid, carbon dioxide, and methane.
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 233 (1980)]**PEER REVIEWED**
Decomposition products: carbon monoxide and carbon dioxide.
[American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991., p. 664]**PEER REVIEWED**
When heated to decomposition it emits acrid smoke and fumes.
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
Hazardous Polymerization:
POLYMERIZES EASILY
[Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 662]**PEER REVIEWED**
Anhydrous, monomeric formaldehyde ... /a dry gas/ is relatively stable at 80-100 deg C but slowly polymerizes at lower temp. Traces of polar impurities such as acids, alkalies, and water qreatly accelerate the polymerization. When liquid formaldehyde is warmed to room temp in a sealed ampule, it polymerizes rapidly with the evolution of heat (63 kJ/mol or 15.05 kcal/mol).
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 233 (1980)]**PEER REVIEWED**
... /Polymerization can be/ inhibited by addition of methanol or of stabilizers such as hydroxypropyl methyl cellulose, methyl and ethyl celluloses or isophthalobisguanamine.
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 244 (1980)]**PEER REVIEWED**
In the presence of small amts of water, formaldehyde gas may slowly trimerize to metaformaldehyde.
[Health and Safety Executive Monograph: Formaldehyde p.2 (1981)]**PEER REVIEWED**
... Formaldehyde and (AZO cmpd) yield exothermic reaction giving off nitrogen gas, (caustics) heat generation and violent polymerization. ... Formaldehyde and (Nitrides) cause heat generation, formation of flammable ammonia gases and violent polymerization. /From table/
[USEPA/ORD; A Method for Determining the Compatability of Haz Wastes (1980) EPA-600/ 2-80-076 as cited in Environment Canada; Tech Info for Problem Spills: Formaldehyde p.84-87 (1985)]**PEER REVIEWED**
Polymerized in aqueous solution to trioxymethylene (retarded by the addition of methanol).
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 524]**PEER REVIEWED**
Immediately Dangerous to Life or Health:
20 ppm; NIOSH considers formaldehyde to be a potential occupational carcinogen.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
Protective Equipment & Clothing:
Respirator selection: (50 ppm) Chemical cartridge respirator with organic vapor cartridge with full facepiece; Gas mask with organic vapor canister (chin-style or front- or back-mounted canister); supplied air respirator with full facepiece, helmet, or hood; self-contained breathing apparatus with full facepiece; (100 ppm): Type C supplied-air respirator operated in pressure-demand or other positive pressure or continuous-flow mode; (escape): Gas mask with organic vapor canister (chin-style or front- or back-mounted canister); self-contained breathing apparatus.
[Sittig, M. Handbook of Toxic and Hazardous Chemicals and Carcinogens, 1985. 2nd ed. Park Ridge, NJ: Noyes Data Corporation, 1985., p. 464]**PEER REVIEWED**
Wear appropriate eye protection to prevent eye contact.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 149]**PEER REVIEWED**
Recommendations for respirator selection. Condition: At concentrations above the NIOSH REL, or where there is no REL, at any detectable concentration: Respirator Class(es): Any self-contained breathing apparatus that has a full facepiece and is operated in a pressure-demand or other positive pressure mode. Any supplied-air respirator that has a full facepiece and is operated in pressure-demand or other positive pressure mode in combination with an auxiliary self-contained breathing apparatus operated in pressure-demand or other positive pressure mode.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 149]**PEER REVIEWED**
Recommendations for respirator selection. Condition: Escape from suddenly occurring respiratory hazards: Respirator Class(es): Any air-purifying, full-facepiece respirator (gas mask) with a chin-style, front- or back-mounted canister providing protection against the compound of concern. Any appropriate escape-type, self-contained breathing apparatus.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 149]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": ... Dispensers of liq detergent /should be available./ ... Safety pipettes should be used for all pipetting. ... In animal laboratory, personnel should ... wear protective suits (preferably disposable, one-piece & close-fitting at ankles & wrists), gloves, hair covering & overshoes. ... In chemical laboratory, gloves & gowns should always be worn ... however, gloves should not be assumed to provide full protection. Carefully fitted masks or respirators may be necessary when working with particulates or gases, & disposable plastic aprons might provide addnl protection. ... Gowns ... /should be/ of distinctive color, this is a reminder that they are not to be worn outside the laboratory. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 8]**PEER REVIEWED**
Preventive Measures:
Formaldehyde is preferably handled in a closed vessels, and if this is impossible the vapors should be removed at the level at which they are evolved. Ventilation must be provided; exposure to concentration above maximum allowed in factory (escape, splashing of liquid, etc) necessitates the workmen wearing complete protective equipment (closed-circuit breathing apparatus, goggles, gloves, etc). Leather and rubber are materials suitable for protection against vapors liquids containing formaldehyde, and clothes, and other articles contaminated by formaldehyde should be copiously washed with water.
[Prager, J.C. Environmental Contaminant Reference Databook Volume 1. New York, NY: Van Nostrand Reinhold, 1995., p. 706-7]**PEER REVIEWED**
PERSONNEL IN CONTACT WITH SOLID MATERIAL CONTAINING FREE FORMALDEHYDE OR WITH CONCN SOLUTIONS OF FORMALDEHYDE, OR EXPOSED TO FORMALDEHYDE VAPORS, SHOULD BE PROTECTED BY SUITABLE EXHAUST OR GENERAL VENTILATION & BE SUPPLIED WITH HAND & ARM PROTECTION & RESP PROTECTIVE EQUIPMENT; BARRIER CREAMS MAY ALSO PROVIDE VALUABLE SKIN PROTECTION.
[International Labour Office. Encyclopedia of Occupational Health and Safety. Vols. I&II. Geneva, Switzerland: International Labour Office, 1983., p. 915]**PEER REVIEWED**
Contact lenses should not be worn when working with this chemical.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 149]**PEER REVIEWED**
SRP: The scientific literature for the use of contact lenses in industry is conflicting. The benefit or detrimental effects of wearing contact lenses depend not only upon the substance, but also on factors including the form of the substance, characteristics and duration of the exposure, the uses of other eye protection equipment, and the hygiene of the lenses. However, there may be individual substances whose irritating or corrosive properties are such that the wearing of contact lenses would be harmful to the eye. In those specific cases, contact lenses should not be worn. In any event, the usual eye protection equipment should be worn even when contact lenses are in place.
**PEER REVIEWED**
If material is not on fire and not involved in a fire: Keep sparks, flames, and other sources of ignition away. Keep material out of water sources and sewers. Build dikes to contain flow as necessary. Use water spray to disperse vapors and dilute standing pools of liquid.
[Association of American Railroads. Emergency Handling of Hazardous Materials in Surface Transportation. Washington, DC: Association of American Railroads, Bureau of Explosives, 1994., p. 516]**PEER REVIEWED**
Personnel protection: Avoid breathing vapors. Keep upwind. ... Do not handle broken packages unless wearing appropriate personal protective equipment. Wash away any material which may have contacted the body with copious amounts of water or soap and water.
[Association of American Railroads. Emergency Handling of Hazardous Materials in Surface Transportation. Washington, DC: Association of American Railroads, Bureau of Explosives, 1994., p. 516]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": Smoking, drinking, eating, storage of food or of food & beverage containers or utensils, & the application of cosmetics should be prohibited in any laboratory. All personnel should remove gloves, if worn, after completion of procedures in which carcinogens have been used. They should ... wash ... hands, preferably using dispensers of liq detergent, & rinse ... thoroughly. Consideration should be given to appropriate methods for cleaning the skin, depending on nature of the contaminant. No standard procedure can be recommended, but the use of organic solvents should be avoided. Safety pipettes should be used for all pipetting. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 8]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": In animal laboratory, personnel should remove their outdoor clothes & wear protective suits (preferably disposable, one-piece & close-fitting at ankles & wrists), gloves, hair covering & overshoes. ... clothing should be changed daily but ... discarded immediately if obvious contamination occurs ... /also,/ workers should shower immediately. In chemical laboratory, gloves & gowns should always be worn ... however, gloves should not be assumed to provide full protection. Carefully fitted masks or respirators may be necessary when working with particulates or gases, & disposable plastic aprons might provide addnl protection. If gowns are of distinctive color, this is a reminder that they should not be worn outside of lab. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 8]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": ... Operations connected with synth & purification ... should be carried out under well-ventilated hood. Analytical procedures ... should be carried out with care & vapors evolved during ... procedures should be removed. ... Expert advice should be obtained before existing fume cupboards are used ... & when new fume cupboards are installed. It is desirable that there be means for decreasing the rate of air extraction, so that carcinogenic powders can be handled without ... powder being blown around the hood. Glove boxes should be kept under negative air pressure. Air changes should be adequate, so that concn of vapors of volatile carcinogens will not occur. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 8]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": Vertical laminar-flow biological safety cabinets may be used for containment of in vitro procedures ... provided that the exhaust air flow is sufficient to provide an inward air flow at the face opening of the cabinet, & contaminated air plenums that are under positive pressure are leak-tight. Horizontal laminar-flow hoods or safety cabinets, where filtered air is blown across the working area towards the operator, should never be used ... Each cabinet or fume cupboard to be used ... should be tested before work is begun (eg, with fume bomb) & label fixed to it, giving date of test & avg air-flow measured. This test should be repeated periodically & after any structural changes. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 9]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": Principles that apply to chem or biochem lab also apply to microbiological & cell-culture labs ... Special consideration should be given to route of admin. ... Safest method of administering volatile carcinogen is by injection of a soln. Admin by topical application, gavage, or intratracheal instillation should be performed under hood. If chem will be exhaled, animals should be kept under hood during this period. Inhalation exposure requires special equipment. ... unless specifically required, routes of admin other than in the diet should be used. Mixing of carcinogen in diet should be carried out in sealed mixers under fume hood, from which the exhaust is fitted with an efficient particulate filter. Techniques for cleaning mixer & hood should be devised before expt begun. When mixing diets, special protective clothing &, possibly, respirators may be required. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 9]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": When ... admin in diet or applied to skin, animals should be kept in cages with solid bottoms & sides & fitted with a filter top. When volatile carcinogens are given, filter tops should not be used. Cages which have been used to house animals that received carcinogens should be decontaminated. Cage-cleaning facilities should be installed in area in which carcinogens are being used, to avoid moving of ... contaminated /cages/. It is difficult to ensure that cages are decontaminated, & monitoring methods are necessary. Situations may exist in which the use of disposable cages should be recommended, depending on type & amt of carcinogen & efficiency with which it can be removed. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 10]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": To eliminate risk that ... contamination in lab could build up during conduct of expt, periodic checks should be carried out on lab atmospheres, surfaces, such as walls, floors & benches, & ... interior of fume hoods & airducts. As well as regular monitoring, check must be carried out after cleaning-up of spillage. Sensitive methods are required when testing lab atmospheres. ... Methods ... should ... where possible, be simple & sensitive. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 10]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": Rooms in which obvious contamination has occurred, such as spillage, should be decontaminated by lab personnel engaged in expt. Design of expt should ... avoid contamination of permanent equipment. ... Procedures should ensure that maintenance workers are not exposed to carcinogens. ... Particular care should be taken to avoid contamination of drains or ventilation ducts. In cleaning labs, procedures should be used which do not produce aerosols or dispersal of dust, ie, wet mop or vacuum cleaner equipped with high-efficiency particulate filter on exhaust, which are avail commercially, should be used. Sweeping, brushing & use of dry dusters or mops should be prohibited. Grossly contaminated cleaning materials should not be re-used ... If gowns or towels are contaminated, they should not be sent to laundry, but ... decontaminated or burnt, to avoid any hazard to laundry personnel. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 10]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": Doors leading into areas where carcinogens are used ... should be marked distinctively with appropriate labels. Access ... limited to persons involved in expt. ... A prominently displayed notice should give the name of the Scientific Investigator or other person who can advise in an emergency & who can inform others (such as firemen) on the handling of carcinogenic substances. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 11]**PEER REVIEWED**
The following engineering controls are recommended to minimize formaldehyde exposure: 1. Local exhaust ventilation should be installed over work stations using formalin or specimens preserved in formalin. 2. Small quantities of formaldehyde should be purchased in plastic containers for ease of handling & safety. 3. Traps should be placed in floor drains. 4. Spill-absorbent bags should be available for emergencies. 5. Engineering controls in hemodialysis units should include (a) isolating the main system from personnel & patients in case of inadvertent spills or (b) disconnecting the dialyzers before the sterilization process is completed. Also, formaldehyde vapors should be prevented from entering the room from the drains serving the main system & the dialysis consoles. The air should be regularly monitored for formaldehyde, & in-service education should be conducted periodically on the effects of formaldehyde.
[Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1216]**PEER REVIEWED**
Stability/Shelf Life:
On standing, especially in the cold, may become cloudy, and on exposure to very low temperature ppt of trioxymethylene formed; in air it slowly oxidizes to formic acid /40% solution/.
[Prager, J.C. Environmental Contaminant Reference Databook Volume 1. New York, NY: Van Nostrand Reinhold, 1995., p. 706]**PEER REVIEWED**
Formaldehyde gas is stable in the absence of water.
[Health and Safety Executive Monograph: Formaldehyde #2 p.2 (1981)]**PEER REVIEWED**
Shipment Methods and Regulations:
No person may /transport,/ offer or accept a hazardous material for transportation in commerce unless that person is registered in conformance ... and the hazardous material is properly classed, described, packaged, marked, labeled, and in condition for shipment as required or authorized by ... /the hazardous materials regulations (49 CFR 171-177)./
[49 CFR 171.2 (7/1/2000)]**PEER REVIEWED**
The International Air Transport Association (IATA) Dangerous Goods Regulations are published by the IATA Dangerous Goods Board pursuant to IATA Resolutions 618 and 619 and constitute a manual of industry carrier regulations to be followed by all IATA Member airlines when transporting hazardous materials.
[IATA. Dangerous Goods Regulations. 42nd Ed. Montreal, Canada and Geneva, Switzerland: International Air Transport Association, Dangerous Goods Regulations, 2001., p. 165]**PEER REVIEWED**
The International Maritime Dangerous Goods Code lays down basic principles for transporting hazardous chemicals. Detailed recommendations for individual substances and a number of recommendations for good practice are included in the classes dealing with such substances. A general index of technical names has also been compiled. This index should always be consulted when attempting to locate the appropriate procedures to be used when shipping any substance or article.
[IMDG; International Maritime Dangerous Goods Code; International Maritime Organization p.3347, 8176-1 (1998)]**PEER REVIEWED**
Storage Conditions:
PROTECT AGAINST PHYSICAL DAMAGE. SEPARATE FROM OXIDIZING & ALKALINE MATERIALS. INDOOR STORAGE SHOULD BE IN AREAS HAVING FLOORS PITCHED TOWARD TRAPPED DRAIN OR IN CURBED RETENTION AREAS. MINIMUM STORAGE TEMP TO PREVENT POLYMERIZATION RANGE FROM 83 DEG F FOR 37% FORMALDEHYDE CONTAINING 0.05% METHYL ALCOHOL TO 29 DEG F FOR FORMALDEHYDE CONTAINING 15% METHYL ALCOHOL.
[National Fire Protection Association. Fire Protection Guide on Hazardous Materials. 9th ed. Boston, MA: National Fire Protection Association, 1986., p. 49-51]**PEER REVIEWED**
Formalin is ... supplied unstabilized or methanol-stabilized. The latter may be stored at room temp without precipitation of solid formaldehyde polymers because it contains 5-10% of methyl alcohol. The uninhibited type must be maintained at a temp of at least 32 deg C to prevent the separation of solid formaldehyde polymers.
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V2 443 (1978)]**PEER REVIEWED**
Inhibition by methanol decreases the minimum storage temp by about 2.3 deg C per wt % methanol for unstabilized soln & about 1.3 deg C per wt % methanol for stabilized solutions. Materials of construction preferred for storage vessels are 304, 316, and 347-type stainless steels or lined carbon steels.
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 244 (1980)]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": Storage site should be as close as practical to lab in which carcinogens are to be used, so that only small quantities required for ... expt need to be carried. Carcinogens should be kept in only one section of cupboard, an explosion-proof refrigerator or freezer (depending on chemicophysical properties ...) that bears appropriate label. An inventory ... should be kept, showing quantity of carcinogen & date it was acquired ... Facilities for dispensing ... should be contiguous to storage area. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 13]**PEER REVIEWED**
Protect containers against physical damage. Separate from oxidizing and alkaline materials. Indoor storage should be in areas having floors pitched toward a trapped drain or in curbed retention areas. Store where temperature range is 16 deg C to 35 deg C. Should not be stored in confined spaces or near open flames. Indoor storage areas should be equipped with automatic sprinklers. Storage tanks should be adequately grounded to discharge static electricity and to reduce other electrical hazard.
[ITII. Toxic and Hazardous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute, 1988., p. 250]**PEER REVIEWED**
Cleanup Methods:
Use fluorocarbon water spray, Cellosize and Hycar to diminish vapors. Sodium carbonate, ammonium hydroxide, or sodium sulfite can neutralize the spill.
[Prager, J.C. Environmental Contaminant Reference Databook Volume 1. New York, NY: Van Nostrand Reinhold, 1995., p. 707]**PEER REVIEWED**
Use universal gel, fly ash, universal sorbent material, or cement powder to absorb the spill.
[Environment Canada; Tech Info for Problem Spills: Formaldehyde p.88 (1985)]**PEER REVIEWED**
Environmental considerations-land spill: Dig a pit, pond, lagoon, holding area to contain liquid or solid material. /SRP: If time permits, pits, ponds, lagoons, soak holes, or holding areas should be sealed with an impermeable flexible membrane liner./ Dike surface flow using soil, sand bags, foamed polyurethane, or foamed concrete. Absorb bulk liquid with fly ash or cement powder. Add sodium bisulfite (NaHSO3).
[Association of American Railroads. Emergency Handling of Hazardous Materials in Surface Transportation. Washington, DC: Association of American Railroads, Bureau of Explosives, 1994., p. 516]**PEER REVIEWED**
Environmental considerations-air spill: Apply water spray or mist to knock down vapors. Combustion products include corrosive or toxic vapors.
[Association of American Railroads. Emergency Handling of Hazardous Materials in Surface Transportation. Washington, DC: Association of American Railroads, Bureau of Explosives, 1994., p. 516]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": A high-efficiency particulate arrestor (HEPA) or charcoal filters can be used to minimize amt of carcinogen in exhausted air ventilated safety cabinets, lab hoods, glove boxes or animal rooms ... Filter housing that is designed so that used filters can be transferred into plastic bag without contaminating maintenance staff is avail commercially. Filters should be placed in plastic bags immediately after removal ... The plastic bag should be sealed immediately ... The sealed bag should be labelled properly ... Waste liquids ... should be placed or collected in proper containers for disposal. The lid should be secured & the bottles properly labelled. Once filled, bottles should be placed in plastic bag, so that outer surface ... is not contaminated ... The plastic bag should also be sealed & labelled. ... Broken glassware ... should be decontaminated by solvent extraction, by chemical destruction, or in specially designed incinerators. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 15]**PEER REVIEWED**
Environmental considerations: water spill: Use natural barriers or oil spill control booms to limit spill travel. Use surface active agent (eg detergent, soaps, alcohols), if approved by USEPA. Inject "universal" gelling agent to solidify encircled spill and increase effectiveness of booms. Add sodium bisulfite (NaHSO3). If dissolved, in region of 10 ppm or greater concentration, apply activated carbon at ten times the spilled amount. Use mechanical dredges or lifts to remove immobilized masses of pollutants and precipitates.
[Association of American Railroads. Emergency Handling of Hazardous Materials in Surface Transportation. Washington, DC: Association of American Railroads, Bureau of Explosives, 1994., p. 516]**PEER REVIEWED**
Approach release from upwind. use water spray to cool and disperse vapors, protect personnel, and dilute spills to form nonflammable mixtures. Stop or control the leak, if this can be done without undue risk. Control runoff and isolate discharged material for proper disposal.
[Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 49-72]**PEER REVIEWED**
Disposal Methods:
Generators of waste (equal to or greater than 100 kg/mo) containing this contaminant, EPA hazardous waste number U122, must conform with USEPA regulations in storage, transportation, treatment and disposal of waste.
[40 CFR 240-280, 300-306, 702-799 (7/1/2000)]**PEER REVIEWED**
Generators of waste (equal to or greater than 100 kg/mo) containing this contaminant, EPA hazardous waste number U122, must conform with USEPA regulations in storage, transportation, treatment and disposal of waste.
[Prager, J.C. Environmental Contaminant Reference Databook Volume 1. New York, NY: Van Nostrand Reinhold, 1995., p. 707]**PEER REVIEWED**
Formaldehyde is a waste chemical stream constituent which may be subjected to ultimate disposal by controlled incineration.
[USEPA; Engineering Handbook for Hazardous Waste Incineration p.2-7 (1981) EPA 68-03-3025]**PEER REVIEWED**
A good candidate for rotary kiln incineration at a temperature range of 820 to 1,600 deg C and residence times of seconds for liquids and gases, and hours for solids. A good candidate for fluidized bed incineration at a temperature range of 450 to 980 deg C and residence times of seconds for liquids and gases, and longer for solids.
[USEPA; Engineering Handbook for Hazardous Waste Incineration p.3-13 (1981) EPA 68-03-3025]**PEER REVIEWED**
Dissolve in a combustible solvent, then spray the soln into the furnace with afterburner. Recommendable methods: Incineration, oxidation, & discharge to sewer. Not recommendable methods: Evaporation & alkaline hydrolysis. Peer-review: Dilute formaldehyde waste with a large amt of water and treat the soln by hypochlorite soln. Concentration of formaldehyde in the soln should be below 2% in order to avoid excess exothermic reaction heat. Formaldehyde is a powerful reducing agent and many oxidants can be used, but may react violently (must be diluted). Alkaline hydrolysis may be dangerous because of exothermic reaction. (Peer-review conclusions of an IRPTC expert consultation (May 1985))
[United Nations. Treatment and Disposal Methods for Waste Chemicals (IRPTC File). Data Profile Series No. 5. Geneva, Switzerland: United Nations Environmental Programme, Dec. 1985., p. 183]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": There is no universal method of disposal that has been proved satisfactory for all carcinogenic compounds & specific methods of chem destruction ... published have not been tested on all kinds of carcinogen-containing waste. ... Summary of avail methods & recommendations ... /given/ must be treated as guide only. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 14]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": ... Incineration may be only feasible method for disposal of contaminated laboratory waste from biological expt. However, not all incinerators are suitable for this purpose. The most efficient type ... is probably the gas-fired type, in which a first-stage combustion with a less than stoichiometric air:fuel ratio is followed by a second stage with excess air. Some ... are designed to accept ... aqueous & organic-solvent solutions, otherwise it is necessary ... to absorb soln onto suitable combustible material, such as sawdust. Alternatively, chem destruction may be used, esp when small quantities ... are to be destroyed in laboratory. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 15]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": HEPA (high-efficiency particulate arrestor) filters ... can be disposed of by incineration. For spent charcoal filters, the adsorbed material can be stripped off at high temp & carcinogenic wastes generated by this treatment conducted to & burned in an incinerator. ... LIQUID WASTE: ... Disposal should be carried out by incineration at temp that ... ensure complete combustion. SOLID WASTE: Carcasses of lab animals, cage litter & misc solid wastes ... should be disposed of by incineration at temp high enough to ensure destruction of chem carcinogens or their metabolites. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 15]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": ... Small quantities of ... some carcinogens can be destroyed using chem reactions ... but no general rules can be given. ... As a general technique ... treatment with sodium dichromate in strong sulfuric acid can be used. The time necessary for destruction ... is seldom known ... but 1-2 days is generally considered sufficient when freshly prepd reagent is used. ... Carcinogens that are easily oxidizable can be destroyed with milder oxidative agents, such as saturated soln of potassium permanganate in acetone, which appears to be a suitable agent for destruction of hydrazines or of compounds containing isolated carbon-carbon double bonds. Concn or 50% aqueous sodium hypochlorite can also be used as an oxidizing agent. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 16]**PEER REVIEWED**
PRECAUTIONS FOR "CARCINOGENS": Carcinogens that are alkylating, arylating or acylating agents per se can be destroyed by reaction with appropriate nucleophiles, such as water, hydroxyl ions, ammonia, thiols & thiosulfate. The reactivity of various alkylating agents varies greatly ... & is also influenced by sol of agent in the reaction medium. To facilitate the complete reaction, it is suggested that the agents be dissolved in ethanol or similar solvents. ... No method should be applied ... until it has been thoroughly tested for its effectiveness & safety on material to be inactivated. For example, in case of destruction of alkylating agents, it is possible to detect residual compounds by reaction with 4(4-nitrobenzyl)-pyridine. /Chemical Carcinogens/
[Montesano, R., H. Bartsch, E.Boyland, G. Della Porta, L. Fishbein, R. A. Griesemer, A.B. Swan, L. Tomatis, and W. Davis (eds.). Handling Chemical Carcinogens in the Laboratory: Problems of Safety. IARC Scientific Publications No. 33. Lyon, France: International Agency for Research on Cancer, 1979., p. 17]**PEER REVIEWED**
The following wastewater treatment technologies have been investigated for formaldehyde: Biological treatment.
[USEPA; Management of Hazardous Waste Leachate, EPA Contract No.68-03-2766 p.E-34 (1982)]**PEER REVIEWED**
The following wastewater treatment technologies have been investigated for formaldehyde: Activated carbon.
[USEPA; Management of Hazardous Waste Leachate, EPA Contract No.68-03-2766 p.E-133 (1982)]**PEER REVIEWED**
Occupational Exposure Standards:
OSHA Standards:
Permissible Exposure Limit: 8 Hr Time-Weighted Avg 0.75 ppm. 15 Min STEL 2 ppm.
[29 CFR 1910.1048(c) (7/1/2000]**PEER REVIEWED**
Threshold Limit Values:
Ceiling limit 0.3 ppm
[American Conference of Governmental Industrial Hygienists. Documentation of Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 2001. Cincinnati, OH. 2001., p. 33]**PEER REVIEWED**
A2: Suspected human carcinogen.
[American Conference of Governmental Industrial Hygienists. Documentation of Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 2001. Cincinnati, OH. 2001., p. 33]**PEER REVIEWED**
NIOSH Recommendations:
Recommended Exposure Limit: 10 Hr Time-Weighted Avg: 0.016 ppm.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
Recommended Exposure Limit: 15 Min Ceiling Value: 0.1 ppm.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
NIOSH considers formaldehyde to be a potential occupational carcinogen.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
NIOSH usually recommends that occupational exposures to carcinogens be limited to the lowest feasible concentration.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
Immediately Dangerous to Life or Health:
20 ppm; NIOSH considers formaldehyde to be a potential occupational carcinogen.
[NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148]**PEER REVIEWED**
Other Occupational Permissible Levels:
Australia (1990): 1 ppm TWA. 2 ppm STEL, probable human carcinogen; Federal Republic of Germany (1991): 0.5 ppm, short-term level 1.0 ppm, 5 min, 8 times/shift; group B, suspected of having carcinogenic potential; danger of sensitization; Sweden (1989): 0.8 ppm, ceiling 1.0 ppm, sensitizer; United Kingdom (1991): 2 ppm, 10-minute STEL 2 ppm
[American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991., p. 683]**PEER REVIEWED**
Emergency Response Planning Guidelines (ERPG): ERPG(1) 1 ppm (no more than mild, transient effects) for up to 1 hr exposure; ERPG(2) 10 ppm (without serious, adverse effects) for up to 1 hr exposure; ERPG(3) 25 ppm (not life threatening) up to 1 hr exposure.
[American Industrial Hygiene Association. The AIHA 2001 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides Handbook. AIHA Press, Fairfax, VA. 2001., p. 25]**PEER REVIEWED**
Manufacturing/Use Information:
Major Uses:
For Formaldehyde (USEPA/OPP Pesticide Code: 043001) ACTIVE products with label matches. /SRP: Registered for use in the U.S. but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses./
[U.S. Environmental Protection Agency/Office of Pesticide Program's Chemical Ingredients Database on Formaldehyde (50-00-0). Available from the Database Query page at http://www.cdpr.ca.gov/docs/epa/epamenu.htm as of May 24, 2001.]**PEER REVIEWED**
FIXATION OF HISTOLOGICAL SPECIMENS & IN ALTERATION OF BACTERIAL TOXINS TO TOXOIDS FOR VACCINES. /SOLN, USP/
[Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 993]**PEER REVIEWED**
AS GERMICIDE ... MAINLY USED IN 2-8% CONCN TO DISINFECT INANIMATE OBJECTS ... .
[Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 970]**PEER REVIEWED**
In the production of fertilizers. As a textile finish, preservative, stabilizer, disinfectant, and antibacterial food additive.
[Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 718]**PEER REVIEWED**
Polyacetal resins, ethylene glycol, pentaerythritol, hexamethylenetetramine, biocide, embalming fluids, reducing agent as in recovery of gold and silver, corrosion inhibitor in oil wells, durable-press treatment of textile fabrics, industrial sterilant, treatment of grain smut, and a versatile chemical intermediate.
[Lewis, R.J., Sr (Ed.). Hawley's Condensed Chemical Dictionary. 13th ed. New York, NY: John Wiley & Sons, Inc. 1997., p. 514]**PEER REVIEWED**
Nickel-plating brightening agent; reagent (Eschweiler-Clarke amine methylation reaction, chloromethylation); latex coagulant; crease-proof textile finishing agent; photographic gelatine hardening agent; preservative (hides, skins); reagent (casein plastic formalisation); crosslinking agent (paper waterproofing)
[Ashford, R.D. Ashford's Dictionary of Industrial Chemicals. London, England: Wavelength Publications Ltd., 1994., p. 440]**PEER REVIEWED**
Used in the manufacture of amino and phenolic resins. Phenol-formaldehyde resins find use as adhesives for binding wood products (particle board, fiber board, and plywood), molding compounds (in electrical, automotive, and kitchen parts), phenolic foam insulation, foundry mold binders, decorative and industrial laminates, and binders for insulating materials. Urea-formaldehyde resins find use as molding compounds, adhesives for paper products. Melamine- formaldehyde resins find use in decorative laminates, thermoset surface coatings, and molding compounds such as dinnerware.
[Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 (1994) 944]**PEER REVIEWED**
Use to make 1,4-butanediol, polyols, acetal resins, hexamethylenetetramine, methylene bis(4-phenyl isocyanate), chelating agents (eg, EDTA and NTA), formaldehyde-alcohol solutions, paraformaldehyde, trioxane, tetraoxane, and many other chemicals.
[Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 (1994) 945]**PEER REVIEWED**
Used as a corrosion inhibitor, hydrogen sulfide scavenger, and biocide in oil production operations such as drilling, waterford, and enhanced oil recovery. Other uses include fungicides, and silage preservatives.
[Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 (1994) 947]**PEER REVIEWED**
CHEM INT FOR PHENOLIC, POLYACETAL & MELAMINE RESINS
**PEER REVIEWED**
Soil sterilant in mushroom houses before planting. /Former use/
[Farm Chemicals Handbook 87. Willoughby, Ohio: Meister Publishing Co., 1987., p. C-121]**PEER REVIEWED**
MEDICATION (VET)
**PEER REVIEWED**
Manufacturers:
Borden Chemical, Inc., 180 East Broad St., Columbus, OH 43215-3799, (614) 225-4000; Production sites: Baytown, TX 77520; Demopolis, AL 36732; Diboll, TX 75941; Fayetteville, NC 28301; Fremont, CA 94538; Geismar, LA 70734; Hope, AR71801; Kent, WA 98031; La Grande, OR 97850; Louisville, KY 40216; Malvern, AR 72104; Missoula, MT 59801; Sheboygan, WI 53081; South Glen Falls, NY 12803; Springfield, OR 97477; Vicksburg, MS 39180; Waverly, VA 23890
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 649]**PEER REVIEWED**
Borden Chemicals and Plastics, Operating Limited Partnership, Highway 73, Geismar, LA 70734, (225) 673-6121; Production site: Geismar, LA 70734
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 649]**PEER REVIEWED**
Capital Resin Corp., 324 Dering Ave., Columbus, OH 43207-2956, (614) 445-7290; Production site: Columbus, OH 43207
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 649]**PEER REVIEWED**
Celanese Ltd., Celanese Chemicals-Americas, 86 Morris Ave., Summit, NJ 07901, (972) 443-4000; Production sites: Bishop, TX 78343; Rock Hill, SC 29730
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 649]**PEER REVIEWED**
D.B. Western, Inc., 1360 Airport Lane, North Bend, OR 97459, (541) 756-0533 Production site: Virginia, MN 55792
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 649]**PEER REVIEWED**
Degussa-Huls Corp., 65 Challenger Rd., Ridgefield Park, NJ 07660, (201) 641-6100. Chemical Group; Production site: Theodore, AL 36590
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 649]**PEER REVIEWED**
DuPont, 1007 Market St., Wilmington, DE 19898, (800) 441-7515. DuPont Specialty Chemicals, Dupont Performance, Specialty, and Fine Chemicals; Production site: La Porte, TX 77571
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 649]**PEER REVIEWED**
Georgia-Pacific Resins, Inc., 55 Park Place, 19th floor, Atlanta, GA 30303, (770) 593-6800; Production sites: Albany, OR 97321; Columbus, OH 43207; Conway, NC 27820; Crosset, AR 71635; Denton, NC 27239; Grayling, MI 49738; Hampton, SC 29924; Houston, TX 77015; Louisville, MS 39339; Lufkin, TX 75901; Russellville, SC 29476; Taylorsville, MS 39168; Vienna, GA 31092; White City, OR 97503
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 649]**PEER REVIEWED**
Geo Specialty Chemicals, Inc., 28601 Chagrin Blvd., Suite 210, Cleveland, OH 44122, (216) 464-5564. TRIMET Products Group; Production site: Allentown, PA 18104
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 650]**PEER REVIEWED**
Hercules Inc., Hercules Plaza, 1313 North Market St., Wilmington, DE 19894- 0001, (302) 594-500. Functional Products Segment, Aqualon Division; Production site: Louisiana, MO 63353
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 650]**PEER REVIEWED**
International Specialty Products, Inc., 1361 Alps Rd., Wayne, NJ 07470-3688, (973) 628-4000; Production site: Texas City, TX 77590
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 650]**PEER REVIEWED**
Neste Resins Corp., 1600 Valley River Drive, Suite 390, Eugene, OR 97401, (541) 687-8840; Production sites: Andalusia, AL 36420; Moncure, NC 27559; Springfield, OR 97477; Toledo, OH 43612; Winnfield, LA 71483
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 650]**PEER REVIEWED**
New Mexico Adhesives LLC, 780 Airport Route, Las Vegas, NM 87701, (505) 425-5932; Production site: Las Vegas, NM 87701
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 650]**PEER REVIEWED**
Perstorp Polyols, Inc., 600 Matzinger Rd., Toledo, OH 43612, (419) 729-5448; Production site: Toledo, OH 43612
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 650]**PEER REVIEWED**
Praxair, Inc., 39 Old Ridgebury Rd., Danbury, CT 06810-5113, (203) 837-2505; Production site: Geismar, LA 70734
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 650]**PEER REVIEWED**
Solutia, Inc., 575 Maryville Centre Drive, P.O. Box 66760, St. Louis, MO 63166-6760, (314) 674-1000; Production site: Alvin, TX 77511
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 650]**PEER REVIEWED**
Wright Chemical Corp., Acme Station, Acme, NC 28446, (910) 251-8952; Production site: Riegelwood, NC 28456
[SRI International. 2000 Directory of Chemical Producers -- United States. SRI Consulting, Menlo Park: CA 2000, p. 650]**PEER REVIEWED**
Methods of Manufacturing:
Historically, formaldehyde has been and continues to be mfr from methanol. During the decades following World War II ... as much as 20% of formaldehyde produced in the USA was made by the vapor phase, non-catalytic oxidation of propane and butanes. ... Today, all of the world's commercial formaldehyde is mfr from methanol and air by an older process using a metal catalyst and a newer one using a metal oxide catalyst. ... In early formaldehyde plants, methanol was oxidized over a copper catalyst, but in recent years this has been almost completely replaced with silver.
[Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 (1994) 934]**PEER REVIEWED**
Oxidation of synthetic methanol or low-boiling petroleum gases such as propane and butane. Silver, copper, or iron-molybdenum oxide are the most common catalysts.
[Lewis, R.J., Sr (Ed.). Hawley's Condensed Chemical Dictionary. 13th ed. New York, NY: John Wiley & Sons, Inc. 1997., p. 514]**PEER REVIEWED**
Methanol (catalytic dehydrogenation)
[Ashford, R.D. Ashford's Dictionary of Industrial Chemicals. London, England: Wavelength Publications Ltd., 1994., p. 439]**PEER REVIEWED**
General Manufacturing Information:
Formaldehyde ... is used in the form of anhydrous monomer, soln, polymers, and derivatives. Anhydrous, monomeric formaldehyde is not avail commercially.
[Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 (1994) 931]**PEER REVIEWED**
U.S. Formaldehyde production capacity in 1989 was 4,310X10+3 tons/yr based on 37% wt formaldehyde (with 2 wt% methanol) /from Table/
[Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 (1994) 941]**PEER REVIEWED**
All information on capacity & demand is reported on a 37% wt formaldehyde basis. Total plant production capacity in the USA in 1978 was 4,086X10+3 tons/year. /From table/
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 240 (1980)]**PEER REVIEWED**
Worldwide production capacity in 1977 was estimated to be over 12.6x10+6 metric tons/yr as 37% by weight formaldehyde.
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 242 (1980)]**PEER REVIEWED**
Formaldehyde, when used as a preservative in shampoos, may interact unfavorably with both fragrance components and color additives.
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V12 91 (1980)]**PEER REVIEWED**
Discontinued in 1987 by Chemical Supply Co, Ltd /Formalin/
[Farm Chemicals Handbook 2001. Willoughby, OH: Meister Publishing Co., P. c 200 (2001)]**PEER REVIEWED**
Worldwide production capacity in 1989 was 15.5X10+6 tons as 37 wt% formaldehyde solution.
[Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 (1994) 939]**PEER REVIEWED**
Formulations/Preparations:
Pure formaldehyde is not avail commercially because of its tendency to polymerize. It is sold as aqueous solutions containing from 37% to 50% formaldehyde by wt & varying amounts of methanol.
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
Marketed under the trade name Formcel, soln in methanol, n-butanol, and isobutanol. ...
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V2 443 (1978)]**PEER REVIEWED**
Aq formaldehyde, known as formalin, is usually 37% by weight of formaldehyde, though more concn soln are available. Formalin is the general-purpose formaldehyde of commerce supplied unstabilized or methanol-stabilized. ... Formaldehyde may also exist in the form of the cyclic trimer trioxane. This is a fairly stable cmpd that does not easily release formaldehyde. ...
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V2 443 (1978)]**PEER REVIEWED**
Grade: Aqueous solutions: 37%, 44%, 50% inhibited (with varying percentages of methanol) or stabilized or unstabilized (methanol-free), also available in solution in n-butanol, ethanol, or urea; USP (37% aqueous solution containing methanol).
[Lewis, R.J., Sr (Ed.). Hawley's Condensed Chemical Dictionary. 13th ed. New York, NY: John Wiley & Sons, Inc. 1997., p. 514]**PEER REVIEWED**
Soluble concentrate; hot fogging concentrate
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium, 11 th ed., British Crop Protection Council, Surrey, England 1997, p. 622]**PEER REVIEWED**
Consumption Patterns:
Worldwide demand for formaldehyde in 1989 was estimated to be about 85-90% of capacity /about 14X10+6 t as 37 wt% formaldehyde soln/
[Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 (1994) 947]**PEER REVIEWED**
Amino resins including urea & melamine, 7.50X10+5 tons. Amino resins molding 5.9X10+4 tons; phenolic resins 6.50X10+5 tons & phenolic molding resins 6.6X10+4 tons; fertilizers 1.80X10+5 tons; textile finishes 6.0X10+4 tons; acetal resins 1.80X10+5 tons; 1.4-butanediol 2.00X10+5 tons; pentaerythritol 1.80X10+5 tons; pyridines 4.0X10+4 tons; methylenediphenyl isocyanate 6.5X10+4 tons; trimethylolpropane 3.5X10+4 tons; & hexamine 1.50X10+5 tons (as 37% formaldehyde, 1978).
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 242 (1980)]**PEER REVIEWED**
CHEM INT FOR UREA-FORMALDEHYDE RESINS, 26.5%; CHEM INT FOR PHENOLIC RESINS, 19.6%; CHEM INT FOR ACETYLENIC CHEMS, 8.4%; CHEM INT FOR POLYACETAL RESINS, 7.9%; CHEM INT FOR PENTAERYTHRITOL, 6.7%; CHEM INT FOR HEXAMETHYLENETETRAMINE, 5.5%; CHEM INT FOR UREA-FORMALDEHYDE CONCENTRATES, 5.2%; CHEM INT FOR METHYLENE DIANILINE, 3.9%; CHEM INT FOR MELAMINE RESINS, 3.6%; CHEM INT FOR CHELATING AGENTS, 2.8%; OTHER, 9.9% (1981).
**PEER REVIEWED**
Worldwide demand for formaldehyde in 1976 was estimated to be about 7.5X10+6 tons or 60% capacity.
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 242 (1980)]**PEER REVIEWED**
During 1985 ... resins going in to adhesives and plastics ... amount to more than 60% of demand ... most of the rest of formaldehyde demand is for use as a chemical intermediate.
[Chem Eng News 63 (5): 14 (1985)]**PEER REVIEWED**
Urea-formaldehyde resins, 27%; phenolic resins, 21%; 1,4-butanediol, 9%; polyacetal resins, 9%; pentaerythritol, 7%; hexamine, 7%; urea-formaldehyde concentrates, 6%; melamine, 4%; MDI, 4%; other, including exports, 6% (1984).
[CHEMICAL PROFILE: Formaldehyde, 1984]**PEER REVIEWED**
CHEMICAL PROFILE: Formaldehyde. Urea formaldehyde resins, 27%; phenolic resins, 21%; acetylenic chemicals, 11%; polyacetal resins, 8%; pentaerythritol, 7%; hexamine, 5.5%; ureal formaldehyde concentrates, 5.5%; melamine resins, 3.8%; MDI, 4.7%; miscellaneous, 5%.
[Kavaler AR; Chemical Marketing Reporter 230 (13): 50 (1986)]**PEER REVIEWED**
CHEMICAL PROFILE: Formaldehyde. Demand: 1985: 5.8 billion lb; 1986: 6 billion lb; 1990 /projected/: 6.63 billion lb.
[Kavaler AR; Chemical Marketing Reporter 230 (13): 50 (1986)]**PEER REVIEWED**
CHEMICAL PROFILE: Formaldehyde. Urea-formaldehyde resins, 25%; phenolic resins, 22%; polyacetal resins, 9%; pentaerythritol, 7%; hexamine, 6%; urea-formaldehyde concentrates, 6%; MDI, 5%; melamine resins, 4%; miscellaneous, 5%.
[Kavaler AR; Chemical Marketing Reporter 236 (12): 54 (1989)]**PEER REVIEWED**
CHEMICAL PROFILE: Formaldehyde. Demand: 6.73 billion lb; 1989: 6.5 billion lb; 1993 /projected/: 7.6 billion lb. (Includes exports but not imports, both of which are negligible).
[Kavaler AR; Chemical Marketing Reporter 236 (12): 54 (1989)]**PEER REVIEWED**
Uses: Urea-formaldehyde (UF) resins (23%), phenolic resins (19%), acetylenic chemicals (12%), polyacetal resins (11%), methylene diisocyanate (MDI) (6%), pentaerythritol (5%), urea-formaldehyde concentrates (4%), hexamethylenetetraamine (HMTA) (4%), melamine resins (4%), misc, including chelating agents, trimethylpropane, pyridine chemicals, nitroparaffin derivatives, textiles treating and trimethylolethane (12%).
[Chemexpo; Chemical Profile: Formaldehyde (June 22, 1998). Available from Database query page at http://www.chemexpo.com/news/PROFile980622.cfm as of May 3, 2001.]**PEER REVIEWED**
U.S. Distribution of formaldehyde production according to uses: (1966) Phenol-formaldehyde resins, 23%; urea-formaldehyde resins, 30%; acetal resins, 2%; pentaerythritol, 12%; hexamethylenetetramine, 6%; urea-formaldehyde concentrates, 5%; ethylene glycol, 14%; others, 8%; (1972) Phenol-formaldehyde resins, 25%; urea-formaldehyde resins, 25%; acetal resins, 8%; 1,4-butanediol, 2%; melamine resins, 8%; pentaerythritol, 7%; hexamethylenetetramine, 6%; urea-formaldehyde concentrates, 5%; others, 14%; (1981) phenol-formaldehyde resins, 20%; urea-formaldehyde resins, 30%; acetal resins, 7%; 1,4-butanediol, 7%; melamine resins, 4%; pentaerythritol, 5%; hexamethylenetetramine, 4%; urea-formaldehyde concentrates, 4%; methylene diisocyanate, 3%; others, 16%; (1984) phenol-formaldehyde resins, 21%; urea-formaldehyde resins, 27%; acetal resins, 9%; 1,4-butanediol, 9%; melamine resins, 4%; pentaerythritol, 7%; hexamethylenetetramine, 7%; urea-formaldehyde concentrates, 6%; methylene diisocyanate, 4%; others, 6%; (1989) Phenol-formaldehyde resins, 22%; urea- formaldehyde resins, 25%; acetal resins, 9%; 1,4-butanediol, 11%; melamine resins, 4%; pentaerythritol, 7%; hexamethylenetetramine, 6%; urea-formaldehyde concentrates, 6%; methylene diisocyanate, 5%; others, 5%.
[Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 (1994) 943]**PEER REVIEWED**
U. S. Production:
(1960) 8.48X10+5 tons/year as 37% formaldehyde
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 241 (1980)]**PEER REVIEWED**
(1965) 1.409X10+6 tons/year as 37% formaldehyde
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 241 (1980)]**PEER REVIEWED**
(1970) 2.008X10+6 tons/year as 37% formaldehyde
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 241 (1980)]**PEER REVIEWED**
(1975) 2.067X10+6 tons/year as 37% formaldehyde
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 241 (1980)]**PEER REVIEWED**
(1977) 2.742X10+6 tons/year as 37% formaldehyde
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 241 (1980)]**PEER REVIEWED**
(1978) 2.948X10+6 tons/year as 37% formaldehyde
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V11 241 (1980)]**PEER REVIEWED**
(1977) 1.01X10+12 G (100% BY WEIGHT SOLN)
**PEER REVIEWED**
(1982) 8.09X10+11 G (100% BY WEIGHT SOLN)
**PEER REVIEWED**
(1983) 3.95X10+12 g (37% by weight soln)
[CHEMICAL PRODUCTS SYNOPSIS: Formaldehyde, 1983]**PEER REVIEWED**
(1988) 6.28X10+9 lb (37% formaldehyde by weight)
[United States International Trade Commission. Synthetic Organic Chemicals- United States Production and Sales, 1988. USITC Publication 1989. Washington, DC: United States International Trade Commission, 1989., p. 15-5]**PEER REVIEWED**
(1990) 6.72 billion lb
[Chem & Engineering News 70 (15): 17 (4/13/92)]**PEER REVIEWED**
(1991) 6.61 billion lb
[Chem & Engineering News 71 (15): 11 (4/12/93)]**PEER REVIEWED**
(1992) 8.28 billion lb
[Chem & Engineering News 72 (15): 13 (4/11/94)]**PEER REVIEWED**
(1993) 7.61 billion lb
[Chem & Engineering News 72 (15): 13 (4/11/94)]**PEER REVIEWED**
U. S. Imports:
(1978) 1.11X10+9 G (INCL SOLNS)
**PEER REVIEWED**
(1983) 7.34X10+9 G (INCL SOLNS)
**PEER REVIEWED**
(1985) 3.87X10+9 g (incl solns)
[BUREAU OF THE CENSUS. U.S. IMPORTS FOR CONSUMPTION AND GENERAL IMPORTS 1985 p.1-580]**PEER REVIEWED**
CHEMICAL PROFILE: Formaldehyde. (1989) 11 million lbs
[Kavaler AR; Chemical Marketing Reporter 236 (12): 54 (1989)]**PEER REVIEWED**
(2002) 140X10+6 lbs (est).
[Chemexpo; Chemical Profile: Formaldehyde (June 22, 1998). Available from Database query page at http://www.chemexpo.com/news/PROFile980622.cfm as of May 3, 2001.]**PEER REVIEWED**
U. S. Exports:
(1978) 1.04X10+10 G (INCL SOLNS)
**PEER REVIEWED**
(1983) 7.80X10+10 G (INCL SOLNS)
**PEER REVIEWED**
(1985) 4.01X10+8 g
[BUREAU OF THE CENSUS. U.S. EXPORTS, SCHEDULE E, 1985 p.2-76]**PEER REVIEWED**
CHEMICAL PROFILE: Formaldehyde. (1998) 19 million lbs
[Kavaler AR; Chemical Marketing Reporter 236 (12): 54 (1989)]**PEER REVIEWED**
(2002) 25X10+6 lbs (est).
[Chemexpo; Chemical Profile: Formaldehyde (June 22, 1998). Available from Database query page at http://www.chemexpo.com/news/PROFile980622.cfm as of May 3, 2001.]**PEER REVIEWED**
Laboratory Methods:
Analytic Laboratory Methods:
... Workplace air samples ... /were/ analyzed by differential pulse polarography. The method was validated over the range of 5.8 - 17.7 mg/cu m. ... Average recovery was 103%. The pooled coefficient of variation or relative standard deviation was 0.08.
[Septon JC, Ku JC; Am Ind Hyg Assoc J 43 (11): 145-52 (1982)]**PEER REVIEWED**
Approximately 20 g of soil, accurately weighed, are collected in a glass jar and dried by the addition of magnesium sulfate. Freon 113 (1,1,2-trichloro-1,2,2-triflouroethane) is used to extract the formaldehyde. The extracts are combined in a 100 ml volumetric flask and the volume taken to 100 ml with Freon. The sample is scanned on a suitable spectrophotometer from 3200 to 2700 cm-1 using matched 1 cm cells. The sample concentration is determined from a calibration curve.
[Amer Water Works Assn; Tech Info for Problem Spills: Formaldehyde p.96 (1985)]**PEER REVIEWED**
Two methods for measuring formaldehyde at ppb levels, the modified pararosaniline and the modified chromotropic acid, were evaluated in a laboratory study. A dynamic double dilution system was used to generate controlled test atmospheres of formaldehyde by the catalytic depolymerization of trioxane. Impinger samples were collected from the sampling manifold and /determined/ accordingly. Both methods demonstrated good precision (3.5% for the pararosaniline and 3.4% for the chromotropic acid) but differed in accuracy and collection efficiency. Accuracy was 87.7 + or - 7.5% for the pararosaniline and 92.5 + or - 4.2% for the chromotropic acid, while collection efficiency was 91.9 + or - 6.9% and 98.7 + or - 4.7%, respectively.
[Petreas M et al; Am Ind Hyg Assoc J 47 (5): 276-80 (1986)]**PEER REVIEWED**
NIOSH Method 2539. Analyte: Formaldehyde. Matrix: Air. Procedure: Gas chromatography, flame ionization detector and gas chromatography/mass spectrometry. For formaldehyde this method has an estimated detection limit of 2 ug aldehyde/sample. The precision/RSD is not determined. Applicability: This is a screening technique to determine the presence of aldehydes and should not be used for quantitation. Interferences: High-boiling naphtha mixtures and mineral spirits may have components with retention times similar to the formaldehydes and may be interferences in the gas chromatographic analysis.
[U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. NIOSH Manual of Analytical Methods. 4th ed. Methods A-Z & Supplements. Washington, DC: U.S. Government Printing Office, Aug 1994., p. ]**PEER REVIEWED**
NIOSH Method 3500. Analyte: Formaldehyde. Matrix: Air. Procedure: Visible absorption spectrometry. For formaldehyde this method has an estimated detection limit of 0.5 ug/sample. The precision/RSD is 0.03 @ 1 to 20 ug/sample. Applicability: The working range is 0.02 to 4 ppm (0.025 to 4.6 mg/cu m) for an 80 liter air sample. Interferences: Phenols, in 8 fold excess over formaldehyde produce a -10% to -20% bias. Little interference is seen from other aldehydes.
[U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. NIOSH Manual of Analytical Methods. 4th ed. Methods A-Z & Supplements. Washington, DC: U.S. Government Printing Office, Aug 1994., p. ]**PEER REVIEWED**
NIOSH Method 2541. Analyte: Formaldehyde. Matrix: Air. Procedure: Gas chromatography hydrogen-air flame ionization detector. For formaldehyde this method has an estimated detection limit of 1 ug/sample. The precision/RSD is 0.0052 @ 38 to 194 ug/sample. Applicability: The working range is 0.24 to 16 ppm (0.3 to 20 mg/cu m) for a 10 liter air sample. Interferences: None have been observed.
[U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. NIOSH Manual of Analytical Methods. 4th ed. Methods A-Z & Supplements. Washington, DC: U.S. Government Printing Office, Aug 1994., p. ]**PEER REVIEWED**
NIOSH Method 5700. Determination of Formaldehyde On Dust (Textile or Wood) by High Performance Liquid Chromatography. This method is applicable to textile and wood dust. Detection limit = 0.08 ug/cu m.
[U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. NIOSH Manual of Analytical Methods. 4th ed. Methods A-Z & Supplements. Washington, DC: U.S. Government Printing Office, Aug 1994., p. ]**PEER REVIEWED**
EPA Method 8015. Direct Injection or Purge-and-Trap Gas Chromatography for the determination of nonhalogenated volatile organics in solid waste. Under the prescribed conditions formaldehyde can be detected using this method. No statistical analysis was determined; specific method performance information will be provided as it becomes available.
[USEPA; Test Methods for Evaluating Solid Waste SW-846 (1986)]**PEER REVIEWED**
EPA Method 8240. Gas Chromatography/Mass Spectrometry for the determination of volatile Organics. This method can be used to quantify most volatile organic compounds including formaldehyde that have boiling points below 200 deg C and are insoluble or slightly soluble in water. The detection limit is not given. Precision and method accuracy were found to be directly related to the concentration of the analyte and essentially independent of the sample matrix.
[USEPA; Test Methods for Evaluating Solid Waste SW-846 (1986)]**PEER REVIEWED**
OSW Method 0011. Sampling of Formaldehyde (and other aldehydes and ketones) from Stack Emissions by Derivatization with 2,4-Dinitrophenyl-Hydrazine. Formaldehyde, and any other aldehydes or ketones present, react with DNPH to yield dinitrophenylhydrazones. These are analyzed by HPLC following extraction and concentration. Detection limit = 1.8 ppb.
[USEPA; EMMI. EPA's Environmental Monitoring Methods Index. Version 1.1. PC# 4082. Rockville, MD: Government Institutes (1997)]**PEER REVIEWED**
OSW Method 0011A. Analysis for Aldehydes and Ketones by High Performance Liquid Chromatography. This method is applicable to aqueous samples, leachates of solid samples (Method 1311), and impinger solutions from Method 0011. Detection limit = 7.2 ug/l.
[USEPA; EMMI. EPA's Environmental Monitoring Methods Index. Version 1.1. PC# 4082. Rockville, MD: Government Institutes (1997)]**PEER REVIEWED**
OSW Method 8315. Determination of Carbonyl Compounds by High Performance Liquid Chromatography (HPLC). This method is applicable to various matrices by derivatization with 2,4-dinitrophenylhydrazine (DNPH). Detection limit = 6.2 ug/l.
[USEPA; EMMI. EPA's Environmental Monitoring Methods Index. Version 1.1. PC# 4082. Rockville, MD: Government Institutes (1997)]**PEER REVIEWED**
OSW Method 8315A-LLE. Determination of Carbonyl Compounds by High Performance Liquid Chromatography (HPLC) Using Liquid-Liquid Extraction. This method is applicable to the determination of free carbonyl compounds in various matrices by derivatization with 2,4-dinitrophenylhydrazine (DNPH). Detection limit = 23 ug/l.
[USEPA; EMMI. EPA's Environmental Monitoring Methods Index. Version 1.1. PC# 4082. Rockville, MD: Government Institutes (1997)]**PEER REVIEWED**
OSW Method 8315A-LSE. Determination of Carbonyl Compounds by High Performance Liquid Chromatography (HPLC) using Liquid-Solid Extraction. This method is applicable to the determination of free carbonyl compounds in various matrices by derivatization with 2,4-dinitrophenylhydrazine (DNPH). Detection limit = 6.2 ug/l.
[USEPA; EMMI. EPA's Environmental Monitoring Methods Index. Version 1.1. PC# 4082. Rockville, MD: Government Institutes (1997)]**PEER REVIEWED**
AOAC Method 897.01. Formaldehyde in Pesticide Formulations by Cyanide Method. Applicable to diluted solutions only. Detection limit not specified.
[Association of Official Analytical Chemists. Official Methods of Analysis. 15th ed. and Supplements. Washington, DC: Association of Analytical Chemists, 1990, p. 226]**PEER REVIEWED**
AOAC Method 898.01. Formaldehyde in Pesticide Formulations by Hydrogen Peroxide Method. Applicable to solutions only. Detection limit not specified.
[Association of Official Analytical Chemists. Official Methods of Analysis. 15th ed. and Supplements. Washington, DC: Association of Analytical Chemists, 1990, p. 226]**PEER REVIEWED**
AOAC Method 931.03: Formaldehyde in Seed Disinfectants by Titrimetric Method. Applicable to the detection of formaldehyde (HCHO) absorbed in inert carrier, e.g., bentonite, talc, charcoal, and sawdust. Detection limit not specified.
[Association of Official Analytical Chemists. Official Methods of Analysis. 15th ed. and Supplements. Washington, DC: Association of Analytical Chemists, 1990, p. 226]**PEER REVIEWED**
EPA Method 554. Determination of Carbonyl Compounds in Drinking Water by Dinitrophenylhydrazine Derivatization and High Performance Liquid Chromatography. This method is used for the determination of selected carbonyl compounds in finished drinking water or raw source water. Detection limit = 8.1 ug/l.
[USEPA; EMMI. EPA's Environmental Monitoring Methods Index. Version 1.1. PC# 4082. Rockville, MD: Government Institutes (1997)]**PEER REVIEWED**
EPA Method 6. Determination of Carbonyl Compounds in Drinking Water by Dinitrophenylhydrazine Derivatization and High Performance Liquid Chromatography. This method is used for the determination of selected carbonyl compounds in finished drinking water or raw source water. Detection limit = 9 ug/l.
[USEPA; EMMI. EPA's Environmental Monitoring Methods Index. Version 1.1. PC# 4082. Rockville, MD: Government Institutes (1997)]**PEER REVIEWED**
AOAC Method 964.21: Formaldehyde in maple syrup spectrophotometric method is not suitable for beet or cane sugars. Detection limit unspecified.
[Association of Official Analytical Chemists. Official Methods of Analysis. 15th ed. and Supplements. Washington, DC: Association of Analytical Chemists, 1990, p. 1037]**PEER REVIEWED**
Sampling Procedures:
NIOSH Method 2539. Analyte: Formaldehyde. Matrix: Air. Sampler: Solid sorbent tube (10% 2-(hydroxy methyl)) pipendine on XAD-2, 20 mg/60 mg. Flow Rate: 0.01 to 0.05 l/min: Sample Size: 5-liters. Shipment: At 25 deg C or lower. Sample Stability: Stable greater or equal to 1 week @ 25 deg C.
[U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. NIOSH Manual of Analytical Methods. 4th ed. Methods A-Z & Supplements. Washington, DC: U.S. Government Printing Office, Aug 1994., p. ]**PEER REVIEWED**
NIOSH Method 3500. Analyte: Formaldehyde. Matrix: Air. Sampler: Filter plus impingers (1 um polytetrafluoroethylene membrane and 2 impingers, each with 20 ml 1% sodium bisulfite solution). Flow Rate: 0.2 to 1 liter/min: Sample Size: 80 liters. Shipment: Transfer samples to flow-density polyethylene bottles before shipping. Sample Stability: 30 days @ 25 deg C.
[U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. NIOSH Manual of Analytical Methods. 4th ed. Methods A-Z & Supplements. Washington, DC: U.S. Government Printing Office, Aug 1994., p. ]**PEER REVIEWED**
NIOSH Method 2541. Analyte: Formaldehyde. Matrix: Air. Sampler: Solid sorbent tube (10% (2-(hydroxymethyl)) piperidine on XAD-2, 120 mg/60 mg). Flow Rate: 0.01 to 0.10 l/min. Sample Size: 10 liters. Shipment: Routine. Sample Stability: 3 weeks @ 25 deg C.
[U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. NIOSH Manual of Analytical Methods. 4th ed. Methods A-Z & Supplements. Washington, DC: U.S. Government Printing Office, Aug 1994., p. ]**PEER REVIEWED**
OSW Method 0100. Sampling for Formaldehyde and Other Carbonyl Compounds in Indoor Air. This method provides procedures for the sampling of various carbonyl compounds in indoor air by derivatization with 2,4-dinitrophenylhydrazine (DNPH) in a silica gel cartridge.
[USEPA; EMMI. EPA's Environmental Monitoring Methods Index. Version 1.1. PC# 4082. Rockville, MD: Government Institutes (1997)]**PEER REVIEWED**
OSW Method 8520. Continuous Measurement of Formaldehyde in Ambient Air. This method is applicable to the continuous measurement of formaldehyde in the 6 to 500 ug/m3 range in ambient air. It is used primarily for nonoccupational exposure monitoring.
[USEPA; EMMI. EPA's Environmental Monitoring Methods Index. Version 1.1. PC# 4082. Rockville, MD: Government Institutes (1997)]**PEER REVIEWED**
Special References:
Special Reports:
Environment Canada; Tech Info for Problem Spills: Formaldehyde (1985)
Chem Indus Inst of Tox Rpts Conf on Formaldehyde Toxicol (1983)
TSCA CHIPs present a preliminary assessment of formaldehyde's potential for injury to human health & the environment (available at EPA's TSCA Assistance Office: (202) 554-1404 or (800) 424-9065)
WHO; Environmental Health Criteria 89: Formaldehyde (1989)
Ma T, Harris MM; Mutat Res 196: 37-59 (1988). Review of the genotoxicity of formaldehyde.
Schlosser O; Disinfection of operating areas by formaldehyde: occupational hazards and their prevention. Literature review of the toxicity of formaldehyde Universit'e Pierre et Marie Curie (Paris VI), Facult'e de m'edecine Daint-Antoine, Paris, France Medical thesis. The study surveyed the conditions under which formaldehyde was used as a disinfectant in the operating theatres of a large hospital in Paris (France), and on the effect this had on the health of hospital staff. Also included are a literature survey of the toxicity of formaldehyde and relevant French legislation.
U.S. Department of Health & Human Services/National Toxicology Program; Tenth Report on Carcinogens. National Institutes of Environmental Health Sciences. The Report on Carcinogens is an informational scientific and public health document that identifies and discusses substances (including agents, mixtures, or exposure circumstances) that may pose a carcinogenic hazard to human health. Formaldehyde (50-00-0) was first listed in the Second Annual Report on Carcinogens (1981) as reasonably anticipated to be a human carcinogen.
[ ]
Synonyms and Identifiers:
Synonyms:
BFV
**PEER REVIEWED**
Dormol
**PEER REVIEWED**
Pesticide Code: 043001
**PEER REVIEWED**
FANNOFORM
**PEER REVIEWED**
FORMALDEHYDE, GAS
**PEER REVIEWED**
FORMALDEHYDE SOLUTION
**PEER REVIEWED**
FORMALIN
**PEER REVIEWED**
FORMALITH
**PEER REVIEWED**
FORMIC ALDEHYDE
**PEER REVIEWED**
FORMOL
**PEER REVIEWED**
FYDE
**PEER REVIEWED**
IVALON
**PEER REVIEWED**
LYSOFORM
**PEER REVIEWED**
METHANAL
**PEER REVIEWED**
METHYL ALDEHYDE
**PEER REVIEWED**
METHYLENE OXIDE
**PEER REVIEWED**
MORBICID
**PEER REVIEWED**
OXOMETHANE
**PEER REVIEWED**
OXYMETHYLENE
**PEER REVIEWED**
SUPERLYSOFORM
**PEER REVIEWED**
Formulations/Preparations:
Pure formaldehyde is not avail commercially because of its tendency to polymerize. It is sold as aqueous solutions containing from 37% to 50% formaldehyde by wt & varying amounts of methanol.
[Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1688]**PEER REVIEWED**
Marketed under the trade name Formcel, soln in methanol, n-butanol, and isobutanol. ...
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V2 443 (1978)]**PEER REVIEWED**
Aq formaldehyde, known as formalin, is usually 37% by weight of formaldehyde, though more concn soln are available. Formalin is the general-purpose formaldehyde of commerce supplied unstabilized or methanol-stabilized. ... Formaldehyde may also exist in the form of the cyclic trimer trioxane. This is a fairly stable cmpd that does not easily release formaldehyde. ...
[Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V2 443 (1978)]**PEER REVIEWED**
Grade: Aqueous solutions: 37%, 44%, 50% inhibited (with varying percentages of methanol) or stabilized or unstabilized (methanol-free), also available in solution in n-butanol, ethanol, or urea; USP (37% aqueous solution containing methanol).
[Lewis, R.J., Sr (Ed.). Hawley's Condensed Chemical Dictionary. 13th ed. New York, NY: John Wiley & Sons, Inc. 1997., p. 514]**PEER REVIEWED**
Soluble concentrate; hot fogging concentrate
[Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium, 11 th ed., British Crop Protection Council, Surrey, England 1997, p. 622]**PEER REVIEWED**
Shipping Name/ Number DOT/UN/NA/IMO:
UN 1198; Formaldehyde solutions, flammable
UN 2209; Formaldehyde solutions, with not less than 25% formaldehyde.
IMO 3.3; Formaldehyde solutions, flammable
IMO 8.0; Formaldehyde solutions, with not less than 25% formaldehyde
Standard Transportation Number:
49 131 68; Formaldehyde solution (liquid) or formalin (flash point more than 141 deg F, in containers over 110 gal)
49 403 64; Formaldehyde solution (liquid) or formalin (flash point more than 141 deg F, in containers of 110 gal or less)
49 131 69; Formaldehyde solution (paste) or formalin (flash point more than 141 deg F, in containers over 110 gal)
49 403 65; Formaldehyde solution (paste) or formalin (flash point more than 141 deg F, in containers of 110 gal or less)
49 131 44; Formaldehyde solution (liquid) or formalin (flash point not more than 141 deg F, in containers over 110 gal)
49 403 41; Formaldehyde solution (liquid) or formalin (flash point not more than 141 deg F, in containers of 110 gal or less)
49 131 45; Formaldehyde solution (paste) or formalin (flash point not more than 141 deg F, in containers over 110 gal)
49 403 42; Formaldehyde solution (paste) or formalin (flash point not more than 141 deg F, in containers of 110 gal or less)
EPA Hazardous Waste Number:
U122; A toxic waste when a discarded commercial chemical product or manufacturing chemical intermediate or an off-specification commercial chemical product or a manufacturing chemical intermediate.